Water doesn’t always freeze at 0 degrees C. Unlike what we have been taught in school, the transformation of liquid water into ice is more nuanced than dropping the temperature down to its freezing point. Supercooled water found in clouds remains in a liquid state at temperatures as low as minus 40 degrees C.
Scientists have even found that completely pure water can remain unfrozen until it’s cooled to temperatures below minus 46 degrees C.
What does water need to freeze?
To freeze, water molecules need to arrange themselves in an ordered way and form a crystalline structure. But ice formation is also kinetically hindered, meaning it requires a bit of extra energy – especially for the first step, called ice nucleation. This energy demand is not small.
To correctly orient themselves to create a crystalline structure, water molecules also need an initiation point, or a nucleus – a place that can serve as a surface on which the ice crystals can grow. This nucleus could be an ice particle or an impurity like dust, minerals, or microorganisms commonly found in water. The lack of these nucleators prevents pure water from freezing, whereas less-pure tap water readily freezes at minus 5 degrees C in our refrigerators.
So pure water struggles to freeze, and this could pose dangers to species adapted to living in cold environs. Then again, life always finds a way. Several microorganisms like bacteria, lichen, and fungi have evolved to manipulate water so that it forms ice more easily. They achieve this with the help of efficient molecular strategies that trigger the nucleation process. This phenomenon is called biological ice nucleation.
In a study published in the Proceedings of the National Academy of Sciences, scientists have explored how fungi can start biological ice nucleation to produce ice on demand.
Biological ice-nucleators are among the most efficient nucleation initiators in nature. However, scientists are yet to fully understand the molecular foundations of this ability.
In the study, a team of scientists from Germany and the U.S. took a closer look at Fusarium acuminatum, a fungal plant pathogen and a known ice-nucleator.
How does life make ice?
Scientists first spotted biological ice nucleation in the 1970s when studying the bacteria Pseudomonas syringae, a plant pathogen that causes multiple diseases in crops. Along with other species of bacteria, P. syringae can start ice formation at temperatures just below the melting point of water (0 degrees C).
These bacteria produce special ice nucleation proteins (INPs) near their cell membranes, which become anchor points for water molecules to start forming ice crystals. Water freezes around the INPs so well it nearly mimics natural ice. A 2016 study found that interactions in certain amino acid sequences within the INPs of P. syringae lead to stronger hydrogen bonds and better structural ordering in water networks. This process moves heat from the water into the bacteria, resulting in quick aggregation of water molecules.
Bacterial INPs are so good at making ice that ski resorts often use a commercial snowmaking product, called Snowmax, which consists of INPs bound to inactivated or dead P. syringae to start the crystallisation process.
In F. acuminatum, however, the scientists found a different mechanism at work.
What does the fungus do differently?
Fungi produce highly efficient ice nucleators that can cause water to start crystallising at temperatures as warm as minus 2 degrees C. Their presence in the soil, the atmosphere, and cloud water-samples has led scientists to suggest they may be able to influence both local and regional weather patterns, if not global.
But unlike bacterial ice-nucleators, the macromolecular structures and interactions in fungal nucleators were still a mystery at the time the study was conducted.
Here, the scientists combined nucleation theory and numerical modelling studies with ice nucleation measurements and physicochemical tests to decipher the chemistry and ice shaping abilities of F. acuminatum.
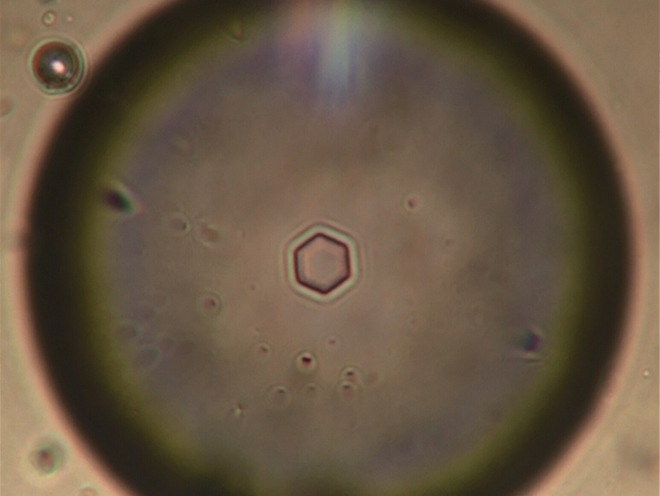
The team found its ice-nucleators to be small extracellular protein subunits made of around 50 amino acids each. F. acuminatum possesses more than a hundred such ice-nucleation proteins that can form functional aggregates outside the fungus’s cells, triggering ice formation.
“In bacteria, the proteins are basically anchored to the cell membrane. You basically can’t extract the proteins without ripping them out of their natural environment, which sometimes makes them unstable,” Konrad Meister, a biochemist at the Max Planck Institute for Polymer Research, Mainz, and Boise State University, Idaho, told this author.
“On the other hand, these fungi are extremely stable because [the proteins are] basically released into the environment and not bound to any membrane.”
Dr. Meister was the study’s corresponding author.
The fungus’s nucleators were also some 25-times smaller than those of the bacteria but still comparably efficient. And because they’re released into the environment, scientists have an opportunity to use the fungal proteins without having to kill the fungi, unlike artificial snow-makers that currently use dead bacteria.
The study concluded that despite the differences in molecular structures of ice-nucleating proteins, nature uses a common strategy to promote high-subzero ice-nucleation temperatures: by assembling the proteins into large, functional aggregates.
“What’s interesting is that fungi and bacteria are very different organisms, right? But somehow, they came up with the same idea and independently evolved to make ice,” Dr. Meister told this author.
Not so tiny applications
The researchers also said they need to further investigate the interplay of water molecules and fungal ice-nucleating proteins, and explore potential applications, especially to make snow-making, cloud-seeding, and cryo-preservation techniques more efficient. If done right, the scientists believe these advancements could save large amounts of power currently required to turn water into ice.
The team is also curious about the ecological advantages of organisms that produce ice-nucleating proteins and their role in cloud formation or rainfall.
“There is a possibility,” Dr. Meister said, “they might be responsible for the beautiful snowfall we experience.”
Sanjukta Mondal is a chemist-turned-science-writer with experience in writing popular science articles and scripts for STEM YouTube channels.
- In a study published, scientists have explored how fungi can start biological ice nucleation to produce ice on demand
- Biological ice nucleators are among the most efficient nucleation initiators in nature
- Fungi produce highly efficient ice nucleators that can cause water to start crystallising at temperatures as warm as minus 2 degrees C







