Pregnancy and childbirth are, at the very least, bizarre. Gestation pushes the body to nurture about a dozen pounds of alien life. Not only does the body grow an eventually independent organism, but it also builds an entirely new organ.
A paper published today in the journal Nature offers the most detailed map so far of how the placenta grows and changes over time, offering insight into how it develops under precise conditions.
A womb of one’s own
This paper, part of a larger project called the Human BioMolecular Atlas Program (HuBMAP), gives us the best view of the placenta, likely since we ourselves were inside one. This organ, and its formation, are indeed mind-boggling.
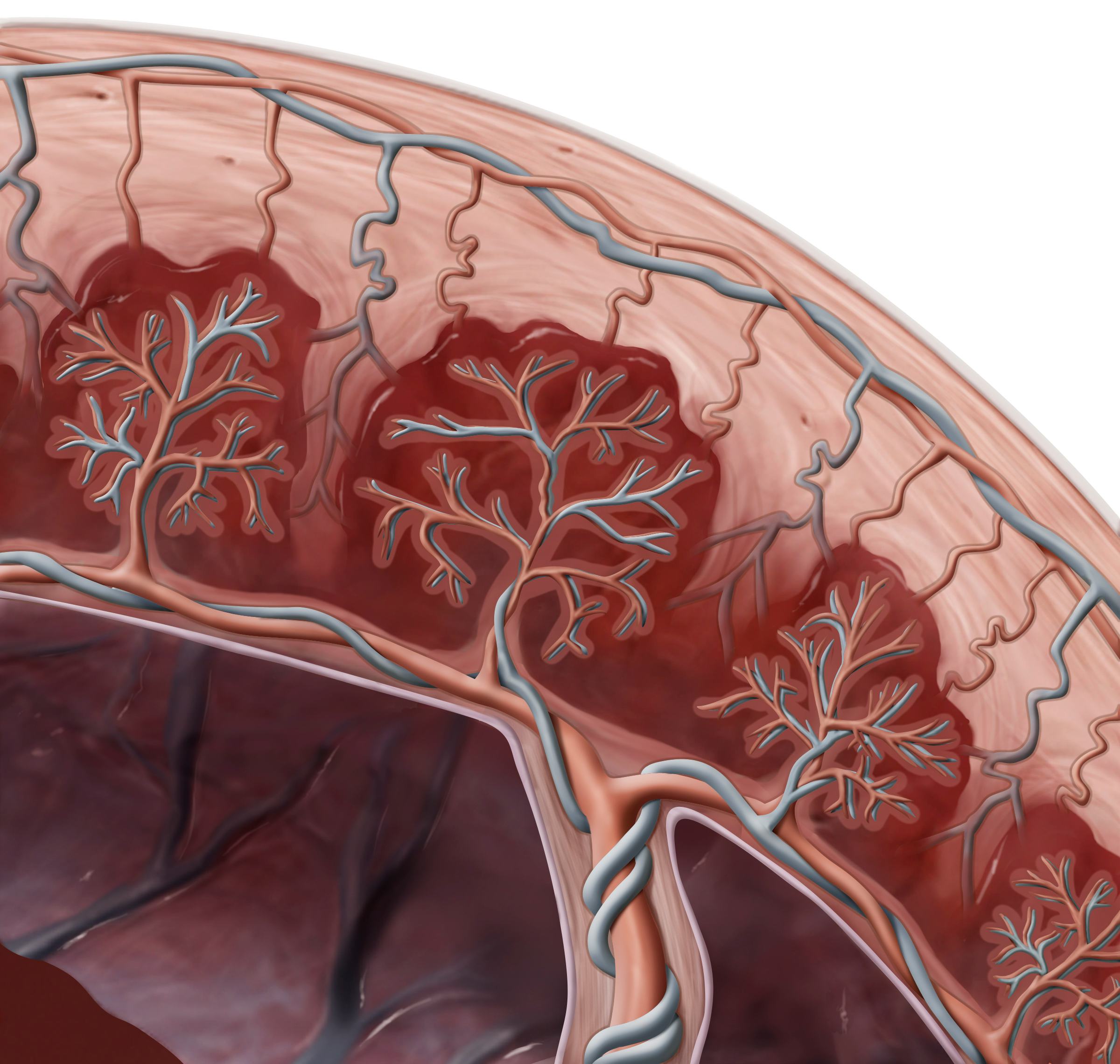
The placenta is a sort of pop-up organ that comes from a fertilized embryo’s outer layer of cells, called the trophectoderm. Once the embryo implants along the uterine wall, the trophectoderm rapidly divides to become the nutritive sac that protects the growing organism until birth. This study offers the most comprehensive timeline so far of cell movement in the thin layer that marks the separation between the parent and the placenta holding the embryo, known as the decidua.
“By getting to know better all the cells in the tissue — their size, their shape, and the proteins they express, through which they communicate with their neighboring cells — we can begin to understand what drives placentation and immune tolerance during pregnancy,” first author Shirley Greenbaum, now an obstetrician-gynecologist at Hadassah University Medical Center, writes to Inverse in an email.
If everything goes perfectly, pregnancy is still a high-risk health condition. If a placenta doesn’t develop just right between the sixth and twentieth weeks of gestation, even more possibilities endanger the parent and fetus. The parent may experience preeclampsia, a condition that can develop after the twentieth week of pregnancy where the parent endures extremely high blood pressure, putting heavy stress on the heart and other organs, and may cause fluid buildup in the lungs.
“This is a crazy process,” senior author Michael Angelo, professor of pathology at Stanford University, tells Inverse. “Because the placenta is genetically fetal, so it's not the same genotype as the mom. In most situations, that would mean the immune system would reject it.” And indeed, that rejection can happen — without the proper balance of immune function, a parent can miscarry.
Three processes, in particular, astonish Greenbaum, and all of them occur in the decidua separating the growing embryo from its parent. First, aggressive immune cells from the parent accumulate in this lining, but they surprisingly don’t attack the genetically foreign embryo and placenta. Next, fetal cells move from the placenta into the decidua, invading the parent’s tissue and blood vessels, which is atypical cell behavior. Third, the parent’s blood vessels literally remodel during the early stages of pregnancy to optimize blood flow to the placenta.
Blood cells, white and red
The placenta’s vascular system and the parent’s immune system are inextricably linked, Angelo says. Blood becomes a vehicle for food for the embryo, but it still carries immune cells on the lookout for potential threats.
“The more blood cells you deliver to the placenta, the more opportunities to activate the immune system,” he tells Inverse. This is because if an immune cell in that deluge of blood decides the embryo and placenta are invasive, it can set off a cascading immune attack simply from providing nutrients.
“If this was any other situation, they'd be like, ‘What the hell is this huge organ that doesn't look like me? We have to destroy it,’” he says. He likens pregnancy to transplanting two pounds of another person’s organs into your body, which of course, would make the immune system freak out.
So the issue becomes how to deliver vast quantities of blood to the embryo without setting off an immune response. On the other hand, turning down immune function is also dangerous because then the parent is at a much higher risk of dying from infection.
The body, however, has figured out a way to hit this equilibrium. Angelo describes a protein called vascular endothelial growth factor (VEGF) that activates during pregnancy to build more blood vessels. However, the protein also signals to immune cells, making them less reactive and less prone to activate the immune response in an area that’s pumping VEGF. While VEGF helps proliferate blood vessel growth, it also gets through to immune cells.
“I think the contribution is that with these new tools, we’re able to look at everything at the same time in a much more comprehensive way to understand how these things are coordinated,” he says.
Greenbaum adds that, surprisingly, the invasive fetal cells (not the parent cells) moving from the placenta to the decidua drove arterial remodeling. “This implies that, perhaps, it is the fetus that is driving the remodeling of its mother's arteries, and not the mother,” Greenbaum writes. Since abnormally remodeled arteries are characteristic of preeclampsia, understanding how these blood highways shift is key to treating patients with this life-threatening disease.
“Evolution is not like a scientist with a protractor measuring things out; it's like a drunk guy with duct tape.”
Placenta flipbook
Using archived tissue samples from 66 patients who chose to have abortions between 6 and 20 weeks of pregnancy, the team painstakingly imaged the placenta at every stage of growth.
To image the arterial growth, they used a new method called multiplexed ion beam imaging by the time of flight, or MIBI-TOF. This process uses light microscopy to label myriad different cells and proteins. From the 66 samples, researchers clocked 588 uterine spiral arteries and categorized them based on their stage of growth.
“It's almost like getting random frames from some point in a movie,” Angelo says. The team had an idea of how to lay out the frames by gestational age but was looking to arrange a timeline of how immune cells and blood vessels change during pregnancy. MIBI-TOF let the team label up to 37 cell markers in each image, which helped them track changes on the molecular level of the tissue sample. For example, they could identify the immune cells in every frame.
Improving childbirth and more
This new understanding of the placenta will certainly help with the treatment of conditions like preeclampsia, but the applications extend beyond childbirth. Surprisingly, these findings can apply to situations that seem completely unrelated, like cancer therapy. Angelo even likens the growth of the placenta to tumor growth.
One of the most encouraging mechanisms, he says, is the redundant systems in place to build a placenta successfully. Existing molecular pathways will even serve completely different purposes, like how VEGF communicates with immune cells in addition to building blood vessels.
“The body rarely does things one way,” Angelo says. “Evolution is not like a scientist with a protractor measuring things out; it's like a drunk guy with duct tape.”







