
There are around 440 nuclear power plants operating in 32 countries around the world, supplying some 10% of the world’s electricity. Another 60 reactors are under construction, and 300 more are proposed.
Australia has only one reactor, used for research and medical purposes. But Australia typically produces more than 5,000 tonnes of uranium each year. This is about 9% of the world’s total.
Uranium mining and processing, and nuclear power plant operations, can produce a range of radioactive elements (called radionuclides). These may be long-lasting hazards if released into the environment. Liquid radioactive wastes present a particular challenge: they often contain a mixture of radionuclides, and few technologies can reliably capture and safely contain these contaminants quickly and efficiently.
We have invented a fast process to capture radionuclides from liquid waste in a clay-like mineral, which can then be baked to form a stable material for disposal and long-term storage. The research is published in Nature Scientific Reports and will soon be presented at the Waste Management Symposium, the world’s largest radioactive waste management conference.
Catching radioactive elements
It has long been known some minerals can capture certain radionuclides. However, this process often involves passing contaminated water through numerous filters packed with these materials.
In contrast, our technology (called EUREECA) uses an approach where a clay-like mineral called a layered double hydroxide is formed within radionuclide-contaminated waters. These minerals are a natural absorbent that can remove a range of radionuclides at once, incorporating these and other contaminants as building blocks in their structure.
This simple approach has many advantages over conventional technologies. In practice, two common industrial chemicals are added to the contaminated water. A reaction occurs in a matter of seconds to produce the layered double hydroxide mineral with the radionuclides trapped inside.

Importantly, the mineral typically comprises less than 0.5% of the mass of the treated water. This means the contaminants become hundreds of times more concentrated.
The mineral is also easily separated from the water using conventional industrial separation techniques.
In studies using wastewater from an Australian uranium mine, the mineral contained up to 1% uranium – a higher concentration than in the mine’s ore. A host of other contaminants were also captured, including a range of radionuclides liberated during mining and associated activities.
Baking for long-term storage
After the contaminants have been captured in the layered double hydroxide mineral, they need to be locked up in perpetuity.
This is the next step of the EUREECA process: baking the mineral to transform it, like pottery in a kiln.
We heated the mineral to more than 1,300℃, similar to that of a Hawaiian lava flow and, with colleagues at Curtin University, analysed how it changed at the atomic level. Several fascinating changes had occurred.
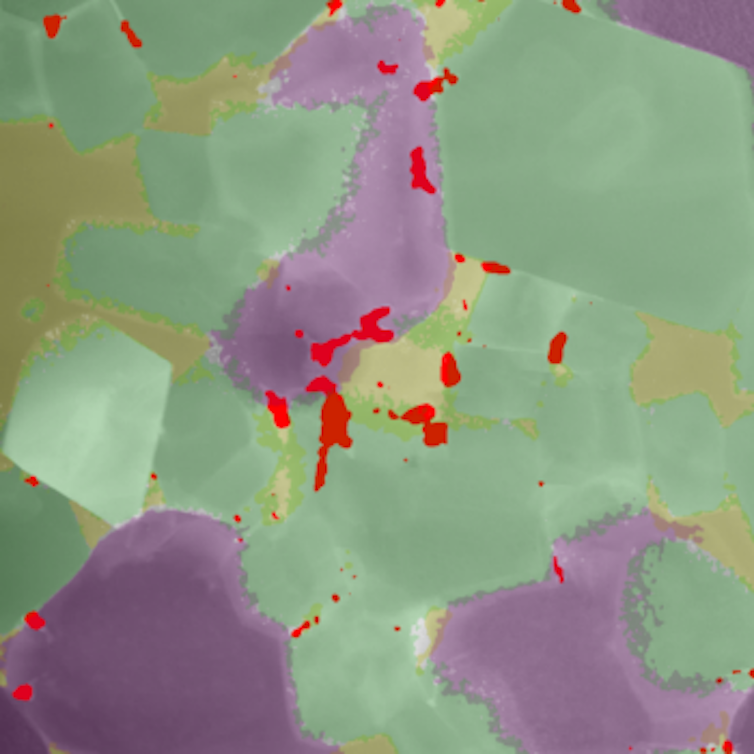
The first was that the layered double hydroxide was transformed into three separate minerals: olivine, periclase and spinel. This is a combination of minerals typically found in the lower mantle, about 2,500km beneath Earth’s surface.
These minerals are not only stable at high temperatures and pressures, but also largely resistant to radiation damage.
When the baked minerals cooled down, we discovered the radionuclides had been concentrated even further. Uranium, thorium, lead and other contaminants were now squeezed into new minerals formed on the microscopically thin boundaries between the olivine, periclase and spinel.
In these boundary regions, the concentration of radionuclides was around 50,000 times greater than in the original uranium-bearing wastewaters.
Easier decontamination
Our process has many potential applications for capture, containment and storage of soluble radioactive wastes in perpetuity. Beyond treating uranium mine wastewater, it could be used to capture and contain radionuclides from medical waste streams.
It would also have been of great use after the Fukushima Daiichi nuclear disaster in 2011, which generated huge amounts of complex liquid waste.
Rather than using multiple steps and substantial, often complex water treatment procedures and infrastructure, the EUREECA technology could have been rapidly deployed to decontaminate the water and remove radionuclides into solid minerals for long-term storage.
Grant Douglas does not work for, consult, or own shares in any company or organisation that would benefit from this article. To support technology commercialisation CSIRO may receive research funding from technology licensees. He has disclosed no relevant affiliations beyond his academic appointment.
This article was originally published on The Conversation. Read the original article.







