
PFAS chemicals (per-and poly fluoroalkyl substances), also known as forever chemicals, are rarely out of the news at the moment. The latest concern about this chemical group is their presence in drinking water in England.
The Royal Society of Chemistry found that the UK’s drinking water standard is not stringent enough to protect us against the dangerous health effects of PFAS. The health risks include links to cancer and fertility problems.
But PFAS are a problem that is not going away anytime soon. Even if we stopped using them in products today, there are already huge amounts of them in the environment and some types of PFAS simply do not degrade. Our research has even found growing evidence of these chemicals in some of the most remote places on Earth, including the Antarctic.
To make matters worse, the PFAS chemicals that do degrade often break down into the more recalcitrant PFAS types, which then cycle around the environment endlessly.
What are PFAS?
Media coverage of the problem can be hard to follow because the PFAS group includes more than 5,000 different chemical substances.
It used to be common to read stories about poly- /perfluoro compounds (PFCs) and poly- /perfluoro alkyl acids (PFAs) rather than PFAS. Even now it is not uncommon to see products like outdoor clothing labelled as PFC free.
But a lot of PFCs are non-toxic and include a lot of widely used medical drugs. PFCs mean drugs with a carbon-fluorine bond, which is not a problem in itself. PFAS are a sub group of PFCs and they are toxic and extremely difficult to break down.
PFAs are a sub-group of PFAS chemicals. Over the last few years research has increasingly highlighted problems with the wider PFAS group than just PFAs. PFAS can be broadly split into two groups: fluoropolymers and non-polymers.
Fluoropolymers
The most well-known fluoropolymer is polytetrafluoroethylene (PTFE) with its trademark name Teflon, first produced by Dupont in the 1940s. PTFE is the non-stick coating to cookware. Its stability to high temperatures, non-reactivity and low friction properties makes it ideal for this application. We can ingest flakes of the polymer, but it is not absorbed by the body so just passes through us.
PTFE film is also used in some types of waterproof, breathable outdoor clothing. The micropores allow the passage of water vapour (from sweat for example) but prevent water droplets seeping through, keeping the wearer dry.
Other fluoropolymers include polyvinylidene fluoride (PVDF) used in sensors and batteries because of its ability to hold an electrical charge. While fluoropolymers are not considered toxic, their production relies on large quantities of other toxic chemicals, like non-polymers. They are better for us because the end product doesn’t bring us into direct contact with non-polymers, but bad news for the environment as that is where the non-polymers will probably end up.
Non-polymers
The non-polymer group has by far the biggest number of substances and their use is myriad – including in food packaging, cosmetics, medical applications, fabric coatings and electronics.
This group also includes refrigerant and cleaning solvents (replacement chemicals to the infamous CFCs banned to protect the ozone). They have high water and oil repellency and chemical stability. For industrial uses chemicals must be stable so that they do their job without reacting or breaking down. If you bought a cosmetic and it lost its colour or wouldn’t spread after a few days, for example, that wouldn’t be good.
Non-polymers also have good thermal stability. This makes it very useful for making food packaging such as pizza boxes and popcorn wrapping.

They are used in a lot of cosmetic products and toiletries because they make the product smoother, water resistant and easier to apply.
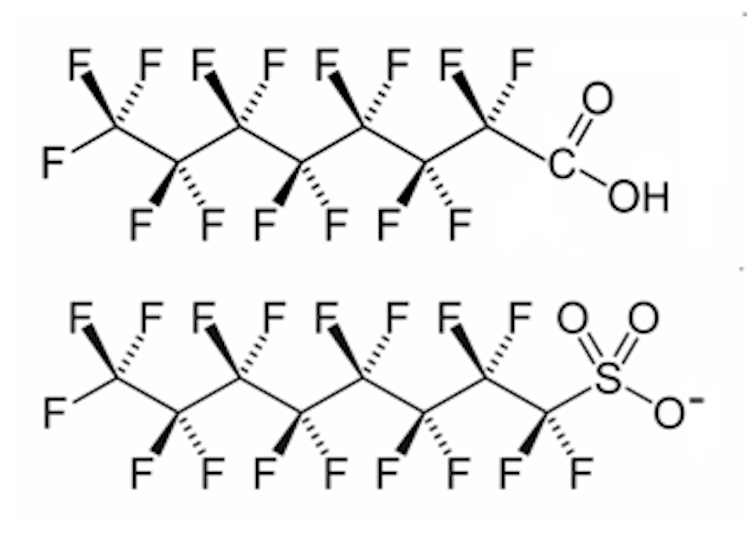
They have an eight-carbon chain (C8) with acidic heads. The longer chain length provides better stability and less friction which means they are useful as processing aids in polymer manufacture.
Since 2000, industry has moved away from C8 chemistry and shifted towards shorter chain length chemicals because of concerns over toxicity of the longer-chain compounds and their harm to the environment.
The shorter chain length does help them break down faster and makes them less toxic. However they don’t perform as well as the longer chain chemicals. So manufacturers use more of them in products and more of them get into the environment.
Toxic effects
The widespread of these chemical groups comes at a cost. PFAS enter the environment through the everyday use and disposal of products that contain them. Emissions from fluoropolymer manufacturing sites is another source.
Domestic and commercial wastewater contains PFAS, which is released into rivers and ocean currents and into remote parts of the planet as evidenced by my team’s recent work investigating high levels of PFAS in Arctic sea ice.
Humans are typically exposed to these chemicals through drinking water, food and household dust. They are a problem for our health because these chemicals have an acid head which tends to interact with and bind to protein molecules in blood. This has knock-on effects on health.
There is strong evidence to demonstrate that exposure to specific PFAS is linked with liver disease, cancer and damages people’s reproductive systems and children’s development. Another area of concern is repression of the immune system and lowered response to vaccines.
Many other countries such as the US and Denmark have revised their drinking water standards. It’s time UK drinking water standards caught up.
Crispin Halsall is a Professor of Environmental Organic Chemistry and receives funding from the Natural Environment Research Council (NERC) for his work on PFAS
This article was originally published on The Conversation. Read the original article.







