Human organs and tissues are made up of millions of microscopic living units called cells. Over their lifespans, these cells accumulate waste products that include unwanted, misfolded and surplus proteins.
Waste management within these cells is a complex and critically important process that is essential for the proper functioning of organs within any given system.
The faulty functioning of cellular waste management machinery can lead to cancer and neurodegenerative diseases. As researchers in immunology and oncology at the Université de Montréal, we want to explain how this process allows cells to adapt to adverse situations.
Proteins are essential
Every cell, tissue or organ contains thousands of different genes. Like a barcode, the genetic information in our DNA is read and translated, enabling the production of thousands of different proteins. Each protein has a precise 3D structure, a specific location and role within a cell type.
Proteins are functional units, similar to tiny machines, that carry out many processes within cells. These processes include the uptake of nutrients to ensure cell survival, cell respiration using oxygen to promote energy production, cell proliferation to replace dead cells and promote organ growth, and cell migration within tissue to place them in the right place at the right time. In short, proteins are responsible for the proper functioning of all cellular processes and allow cells to coexist in harmony within an organism.
Each of our cells has exactly the same set of genes, but each cell type has a unique protein profile. For example, one type of protein may be present and active in brain cells but absent in kidney or muscle cells. A protein might be essential for one organ but not for another, and their presence or absence within the cell is governed by a dynamic balance, orchestrated by mechanisms that regulate protein production and elimination.
How cells decide which proteins to discard
Over the past few decades, researchers have learned a great deal about how proteins are produced from genes via messenger RNA translation. This process involves a structure called the ribosome, the factory for protein production.
Once produced, some proteins must be eliminated, either because of they are misfolded or because they have become redundant. Protein degradation is a highly co-ordinated and complex cellular process.
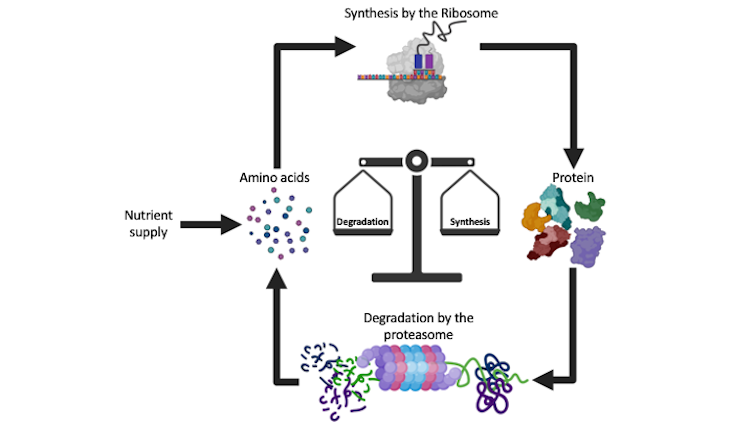
Our lab, among others, is interested in understanding how cells make the initial decision to eliminate proteins and then proceed to destroy them. To ensure their removal, cells set up a complex process of quality control and decision-making that results in protein ubiquitination. Ubiquitination is an essential process that consists of binding a small protein, called ubiquitin, to various unwanted protein targets. It occurs in all cells in the body.
The ubiquitination stamp allows the proteasome to recognize unwanted proteins and sort them for removal. The proteasome is a tiny cylindrical chamber made up of many specialized proteins, which act like molecular scissors to shred proteins. As an essential protein complex, there are multiple copies of the proteasome in all living cells.
The proteasome is responsible for the rapid and highly specific breakdown of unwanted, misfolded or surplus proteins. This process is extremely important for the proliferation and proper functioning of cells.
The aberrant or reduced degradation of cellular proteins can lead to a variety of diseases, including cancer and brain diseases. However, the exact mechanisms underlying the normal functions and pathological alterations associated with the proteasome are still poorly understood.
Cells react to lack of nutrients
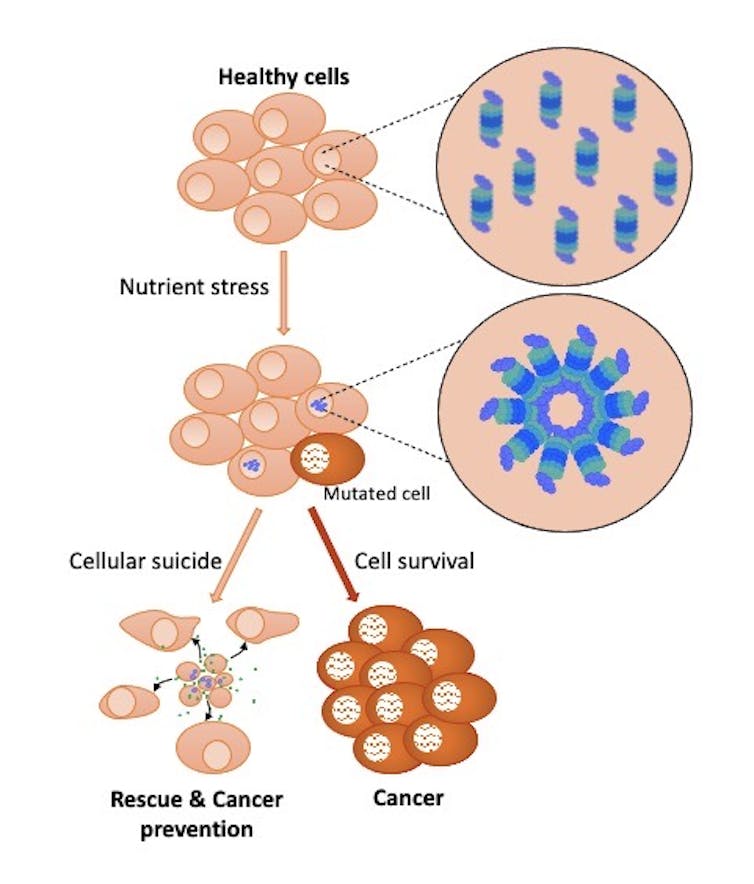
We recently discovered that when the body is nutrient deprived, proteasomes assemble in the nucleus of cells to form large structures called “bodies” or “foci.” This aggregation of proteasomes can be observed in various cell types and is a general cellular response to nutrient deprivation.
Specifically, this phenomenon only occurs when cells are deprived of amino acids, the necessary building blocks of proteins. Consequently, there’s a tightly controlled balance between the supply of amino acids for protein synthesis and their break down by the proteasome.
The formation of proteasome foci amplifies this degradation process during periods of nutrient deprivation. Interestingly, our study also found that these foci promote cell death during severe nutrient stress, where the cell triggers molecular mechanisms that lead to its destruction, a form of cellular suicide. Although this cell death is detrimental to individual cells, the outcome could be beneficial for overall cell population that forms the tissues and organs.
In fact, the death of some cells in an organ, in response to nutrient deficiency, could initially decrease the competition between cells for limited resources. The release of cellular components, in particular nutrients, during cell death could help nearby cells survive. In addition, dying cells could send signals to summon specialized rescue cells to repair tissues.
We also found that some cells present in tumours have a reduced ability to form proteasome foci following nutrient deprivation, suggesting that these cells have acquired resistance to stress. The formation of these foci, in normal cells, could be a defence mechanism that promotes the death of cells that have undergone drastic changes caused by the absence of nutrients.
In this respect, inducing cellular suicide by forming proteasome foci in cells that have undergone changes that promote cancer development could be an interesting new approach to prevent cancer.
Now that researchers have a better understanding of what affects proteasome function, they could target it for personalized cancer treatment, which requires the knowledge of all the molecular disruptors of cancer cells.
It is possible that cells that escaped death as a result of nutrient stress have nevertheless accumulated changes in their functioning that could make them vulnerable. We are currently working on this hypothesis.
Malik Affar co-authored this article and helped produce the graphics.
El Bachir Affar received funding from the Canadian Institutes of Health Research (CIHR).
Clémence Messmer received funding from the Canadian Institutes of Health Research (CIHR).
This article was originally published on The Conversation. Read the original article.







