The Food and Drug Administration is facing a federal investigation into whether it correctly followed its policies and procedures in the contamination and recall of baby formula that lead to a national shortage and the potential deaths of infants.
The Department of Health and Human Services announced on Thursday that it would specifically review whether or not the FDA correctly conducted inspections of Abbott's Sturgis, Mich., manufacturing facility at the center of the recall and if it correctly oversaw Abbott's initiation of the infant formula recall.
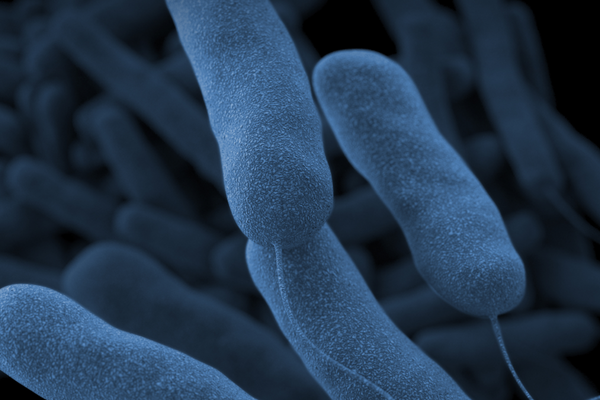
In February, Abbott issued a recall of some of its baby formula products after one of the company's plants was suspected of contamination of a bacteria called Cronobacter sakazakii, which infected four infants, two who died. The voluntary recall included certain lots of Similac, Similac Alimentum and EleCare formula products.
The recall resulted in stores across the country facing empty baby formula shelves.
The move to conduct an investigation was applauded by Rep. Rosa DeLauro, D-Conn., who called on the FDA in March to provide answers as to why there was delayed action and the process for inspections.
"I commend the OIG for taking this critical step to get answers as to why this unreasonable delay was allowed to occur — especially when a product we feed to our babies was at the center of allegations of wrongdoing," DeLauro said in a statement. "I hope the OIG moves speedily to uncover answers so that we can prevent this crisis from potentially happening again."
Finger pointing over who knew what when
In May the Biden administration invoked the Defense Production Act to speed up domestic production of infant formula and put in place measures along with HHS to increase the import of millions of bottles of baby formula from other countries. The administration also issued flexibilities in the WIC program, the government program that largely uses Abbott for its contracts.
But questions have remained over what government officials were communicated about the potential food security threat and why action was not taken sooner.
During a Thursday afternoon press briefing, White House press secretary Karine Jean-Pierre was unable to tell reporters who first told President Biden about the potential shortages.
"He's briefed on countless priorities. There are regular channels — he is briefed by his senior White House staff," Jean-Pierre said, earlier noting that the FDA was too slow to act. "I am not going to confirm who it was."
Recent congressional hearings have also done little to shed light on the communication snafus. A whistleblower report mailed in October, detailing food safety inspection problems at the Michigan plant, failed to reach then-acting Commissioner Janet Woodcock and other FDA leaders till February.
In testimony before the House Appropriations Committee, FDA Commissioner Robert Califf blamed "mailroom issues."