Electrons moving around in a molecule might not seem like the plot of an interesting movie. But a group of scientists will receive the 2023 Nobel Prize in physics for research that essentially follows the movement of electrons using ultrafast laser pulses, like capturing frames in a video camera.
However, electrons, which partly make up atoms and form the glue that bonds atoms in molecules together, don’t move around on the same time scale people do. They’re much faster. So, the tools that physicists like me use to capture their motion have to be really fast – attosecond-scale fast.
One attosecond is one billionth of a billionth of a second (10-18 second) – the ratio of one attosecond to one second is the same as the ratio of one second to the age of the universe.
Attosecond pulses
In photography, capturing clear images of fast objects requires a camera with a fast shutter or a fast strobe of light to illuminate the object. By taking multiple photos in quick succession, the motion of the object can be clearly resolved.
The time scale of the shutter or the strobe must match the time scale of motion of the object – if not, the image will be blurred. This same idea applies when researchers attempt to image the ultrafast motion of electrons. Capturing attosecond-scale motion requires an attosecond strobe. The 2023 Nobel laureates in physics made seminal contributions to the generation of such attosecond laser strobes, which are very short pulses generated using a powerful laser.
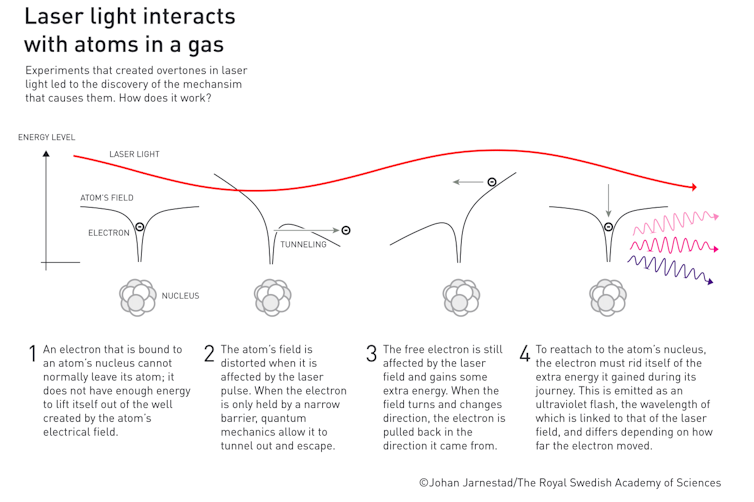
Imagine the electrons in an atom are constrained within the atom by a wall. When a femtosecond (10-15 second) laser pulse from a high-powered femtosecond laser is directed at atoms of a noble gas such as argon, the strong electric field in the pulse lowers the wall.
This is possible because the laser electric field is comparable in strength to the electric field of the nucleus of the atom. Electrons see this lowered wall and pass through in a bizarre process called quantum tunneling.
As soon as the electrons exit the atom, the laser’s electric field captures them, accelerates them to high energies and slams them back into their parent atoms. This process of recollision results in creation of attosecond bursts of laser light.
Attosecond movies
So how do physicists use these ultrashort pulses to make movies of electrons at the attosecond scale?
Conventional movies are made one scene at a time, with each instant captured as a frame with video cameras. The scenes are then stitched together to form the complete movie.
Attosecond movies of electrons use a similar idea. The attosecond pulses act as strobes, lighting up the electrons so researchers can capture their image, over and over again as they move – like a movie scene. This technique is called pump-probe spectroscopy.
However, imaging electron motion directly inside atoms is currently challenging, though researchers are developing several approaches using advanced microscopes to make direct imaging possible.
Typically, in pump-probe spectroscopy, a “pump” pulse gets the electron moving and starts the movie. A “probe” pulse then lights up the electron at different times after the arrival of the pump pulse, so it can be captured by the “camera,” such as a photoelectron spectrometer.
The information on the motion of electrons, or the “image,” is captured using sophisticated techniques. For example, a photoelectron spectrometer detects how many electrons were removed from the atom by the probe pulse, or a photon spectrometer measures how much of the probe pulse was absorbed by the atom.
The different “scenes” are then stitched together to make the attosecond movies of electrons. These movies help provide fundamental insight, with help from sophisticated theoretical models, into attosecond electronic behavior.
For example, researchers have measured where the electric charge is located in organic molecules at different times, on attosecond time scales. This could allow them to control electric currents on the molecular scale.
Future applications
In most scientific research, fundamental understanding of a process leads to control of the process, and such control leads to new technologies. Curiosity-driven research can lead to unimaginable applications in the future, and attosecond science is likely no different.
Understanding and controlling the behavior of electrons on the attosecond scale could enable researchers to use lasers to control chemical reactions that they can’t by other means. This ability could help engineer new molecules that cannot be created with existing chemical techniques.
The ability to modify electron behavior could lead to ultrafast switches. Researchers could potentially convert an electric insulator to a conductor on attosecond scales to increase the speed of electronics. Electronics currently process information at the picosecond scale, or 10-12 of a second.
The short wavelength of attosecond pulses, which is typically in the extreme-ultraviolet, or EUV, regime, may see applications in EUV lithography in the semiconductor industry. EUV lithography uses laser light with a very short wavelength to etch tiny circuits on electronic chips.

In the recent past, free-electron lasers such as the Linac Coherent Light Source at SLAC National Accelerator Laboratory in the United States have emerged as a source of bright X-ray laser light. These now generate pulses on the attosecond scale, opening many possibilities for research using attosecond X-rays.
Ideas to generate laser pulses on the zeptosecond (10-21 second) scale have also been proposed. Scientists could use these pulses, which are even faster than attosecond pulses, to study the motion of particles like protons within the nucleus.
With numerous research groups actively working on exciting problems in attosecond science, and with 2023’s Nobel Prize in physics recognizing its importance, attosecond science has a long and bright future.
Niranjan Shivaram receives funding from the National Science Foundation and U.S. Department of Energy.
This article was originally published on The Conversation. Read the original article.







