
Figuring out what species live in an ecosystem, and which ones are rare or just good at hiding is an essential way to understand and care for them. Until now, it’s been very labour intensive.
But now we can do it differently. Take a sample from the ocean and match tiny traces of DNA in the water with the species living there.
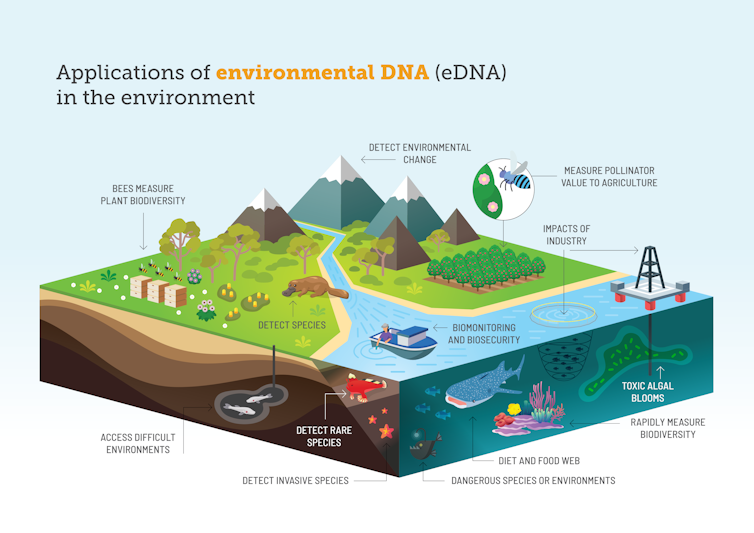
It’s not science fiction – it’s environmental DNA sampling. This approach opens the door to rapid, broad detection of species. You can find if pest species have arrived, tell if a hard-to-find endangered species is still hanging on, and gauge ecosystem health.
Because eDNA testing is still new, there are questions about its strengths and weaknesses and how it can best be used. For instance, we can tell if extremely rare freshwater sawfish are present in a Northern Territory river – but not how many individual fish there are.
Today CSIRO released a roadmap created through consultation with many experts to show how eDNA technologies can be best integrated into marine monitoring at a large scale – and what the future holds.

How does eDNA sampling work?
Deoxyribonucleic acid (DNA) is a very special molecule. It acts as the code for all life on Earth, holding the cellular instructions to make everything from a beetle to a human. Because DNA is unique to each species, it’s like a product barcode in a supermarket.
As animals and plants live their lives, they shed fragments of their DNA into their environment through dead skin, hair, saliva, scat, leaves or pollen. These traces make up environmental DNA. There’s enough DNA in water and even air to tell species apart.
The real power of eDNA sampling is how broad a net it casts. With one sample, we can detect anything living, from bacteria to whales, and in potentially every environment with life, from the deep sea to underground caves.
Importantly, this method lets scientists detect species even if we can’t see or capture them. This comes in handy when working with rare or very small species, or when working in environments such as murky water where it is impossible to see or catch them. It will, for example, make it easier to study critically endangered pipefish in estuaries.
To date, much eDNA research has focused on detecting species in water, because it’s relatively easy to collect, concentrate and extract eDNA from liquids. But we now know we can produce species lists based on the eDNA in soil, scat, honey, or even the air.
How do scientists actually measure eDNA?
Typically, you collect samples, perform molecular analysis and interpret results.
Collect samples: Scientists collect a sample from the environment. This can be water, soil, or virtually any environmental substrate which might contain eDNA. We then process the sample to concentrate and stabilise the DNA. You might collect two litres of water with a bucket, filter it and then freeze or chemically stabilise the eDNA coating the filter.
Molecular analysis: The first step in the lab is to purify DNA from a sample. The next step depends on your goal. If you want to detect a single species, you would generally use a technique called quantitative polymerase chain reaction (qPCR), similar to how you test for COVID.
But to detect whole communities of species, you have to use high-throughput DNA sequencing. Where detecting a single species with eDNA takes only a few days days, completing the labwork for species communities can take weeks to months. At the end, you arrive at a long list of thousands or even millions of DNA barcode sequences.
Interpreting results: Single species interpretation is simple. Was DNA from your species of interest present or not? But when the goal is to identify multiple species, scientists use DNA reference libraries to link the DNA barcodes detected in the sample back to individual species.
This works well – but only if we already have the species listed in these libraries. If not, you can’t detect it with eDNA methods. That means eDNA can’t be used to detect new species or those only known from photos and videos.
Why does eDNA matter? Look at marine parks
Australia boasts one of the world’s largest and most biodiverse networks of marine parks. But as ocean life reels from climate change, overfishing and plastic pollution, it’s certain the oceans of the future will look very different to that of today.
Gauging impact to support evidence-based decisions across such a vast, diverse and remote area poses challenges difficult to solve with standard hands-on survey methods like like diving, video or trawling.
That’s where eDNA methods can help, offering a powerful, non-destructive, cost-effective and quick form of monitoring that can complement other techniques.
eDNA means we can fine-tune monitoring for specific purposes, such as detecting pests, endangered, or dangerous species.
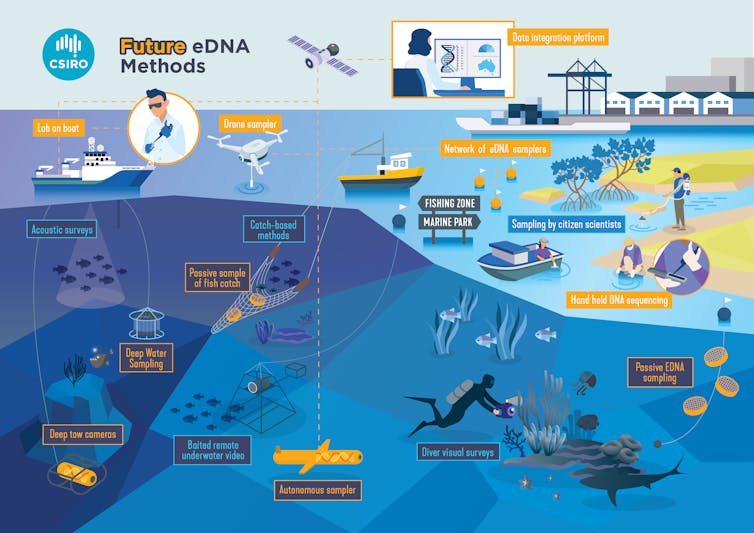
This is just the start. Imagine a future where eDNA data could be collected from the most remote oceans by autonomous vehicles, analysed by the drone or on board a research vessel, and integrated with other monitoring data so marine managers and the public can see near-real time data about the condition of the ocean.
Science fiction? Not any more.
Maarten De Brauwer receives funding from the CSIRO and the National Geographic Society. He is a board member at the Southern eDNA Society.
Oliver Berry receives funding from the CSIRO, the Australian Government, and the Minderoo Foundation. He is a board member of the Southern eDNA Society (Australia and New Zealand's professional society for eDNA scientists and other stakeholders).
This article was originally published on The Conversation. Read the original article.







