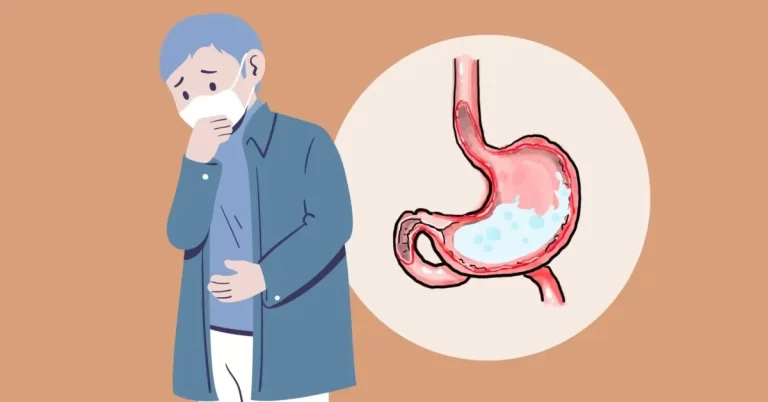
Ozempic, also known as semaglutide, is a medication used to treat type 2 diabetes. Recently, it has been in the spotlight due to lawsuits being filed against its manufacturer, Novo Nordisk. These lawsuits claim that Ozempic has been linked to a serious gastrointestinal condition called gastroparesis.
Gastroparesis is a disorder in which the stomach takes longer than usual to empty its contents into the small intestine. This can cause symptoms such as nausea, vomiting, bloating, and abdominal pain. In severe cases, it can lead to malnutrition and dehydration.
The connection between Ozempic and Gastroparesis has raised concerns among patients who have been prescribed the medication.
So, what exactly is product liability, and why is it relevant in this situation?
Understanding Product Liability
Product liability refers to the legal responsibility of a manufacturer or seller for any injuries or damages caused by their product. For a product liability lawsuit to be successful, three elements must be present:
- Defect: The product must have a defect that makes it unreasonably dangerous.
- Injury: The defect in the product must have caused injury or harm to the consumer.
- Causation: There must be a direct link between the defect and the injury sustained by the consumer.
In the case of Ozempic, the lawsuits are claiming that there is a defect in the medication that causes gastroparesis as well as other serious side effects. This defect is ultimately what led to the injuries and harm suffered by patients who took the medication.
Failure to Warn
One specific type of product liability claim being made against Novo Nordisk regarding Ozempic is failure to warn. Failure to warn, also known as inadequate warning or inadequate label, occurs when a manufacturer fails to provide sufficient information about potential risks or dangers associated with their product.
In the case of Ozempic, the lawsuits claim that Novo Nordisk did not adequately warn patients and healthcare providers about the potential risk of developing gastroparesis while taking the medication.
For a failure to warn claim to be successful, three elements must be present:
- Duty to Warn: The manufacturer is legally obligated to warn consumers about any potential risks associated with using their product.
- Failure to Warn: The manufacturer failed to provide adequate warning or information about the potential risks of using the product.
- Causation: The lack of warning directly led to the injury or harm suffered by the consumer.
Why Failure to Warn Matters
Failure to warn is essential to product liability because it holds manufacturers accountable for providing accurate and complete information about their products. Consumers have the right to make informed decisions about what they put into their bodies, and without proper warning, they may not be aware of potential risks.
Here are some key reasons why failure to warn matters:
- Injury and Harm: Inadequate warnings can result in accidents, injuries, and even fatalities. Consumers may not be aware of potential dangers, leading to misuse or improper handling of products.
- Healthcare Provider Knowledge: Healthcare providers rely on accurate and complete manufacturer information to make informed medication prescription decisions. Failure to warn can put patients at risk and create challenges for healthcare professionals.
- Legal Liability: Manufacturers have a legal responsibility to warn consumers about the potential dangers of their products. Failure to do so can result in costly lawsuits and damage their reputation and credibility.

The Importance of Accurate and Complete Information
The failure to warn allegations in the Ozempic lawsuit highlights the importance of providing accurate and complete medication information. This applies not only to pharmaceutical companies but also to all manufacturers.
Consumers have the right to know what they are buying and using, and it is up to manufacturers to provide that information. Without proper warning, consumers may be at risk for serious health consequences.
As more information comes to light regarding Ozempic and its potential link to gastroparesis, it becomes increasingly clear why product liability and failure to warn are essential considerations. Manufacturers must take responsibility for their products and ensure that consumers are fully informed about any potential risks associated with them.
If you or a loved one has experienced adverse side effects from taking Ozempic, it is essential to consult a legal professional practicing product liability. They can help you understand your rights and options for seeking compensation for any injuries or damages caused by the medication.