This article by Elisabetta Fato was originally published on Microdose and appears here with permission.
In the last decades, there has been a growing interest in psychedelics for the treatment of mood and behavioral disorders, among which are substance use disorders.
Lately, the psychedelic compound ketamine is gaining particular attention for the treatment of alcohol use disorder (AUD), with clinical studies being conducted to explore its potential. In this article, we’re going to get a closer look at phase 2b ketamine trial for alcohol dependence, soon going into phase 3, conducted by the biotech company Awakn Life Sciences.
But before diving into the current clinical developments, let’s first discuss some key points: the importance of new treatments for alcohol addiction, why ketamine therapy looks so promising, and the preclinical studies supporting its potential to treat alcohol use disorder.
Epidemiology of alcohol addiction
Alcohol abuse causes millions of deaths worldwide, by producing physical damage as well as triggering mental and behavioural conditions. In the US, for instance, almost 7% of adults suffer from alcohol use disorder, while nearly 30% of the American population has experienced problems with alcohol during their lives. Given its prevalence across the world and its impact on healthcare, it is of major importance to establish an effective treatment. Given the limits of existing solutions like behavioural therapy and medications such as naltrexone, which sometimes present its own problems, new therapies are needed to face this crisis and one might be ketamine.
Ketamine psychedelic therapy
The mechanism behind the effectiveness of psychedelics for the treatment of substance misuse is based on reduction in the activity of a brain network known to be overactivated in mood disorders, called the Default Mode Network (DMN). DMN is associated with ruminative and self-reflective states, overactivated depression and other mental diseases. Consequently, disrupting its activity in mood disorder patients was shown to reduce depressive symptoms.
The resulting psychological effects, including “ego dissolution” — increased spiritual and conscious awareness and so forth — lead to a significant reduction of the symptoms seen in mood and substance use disorders.
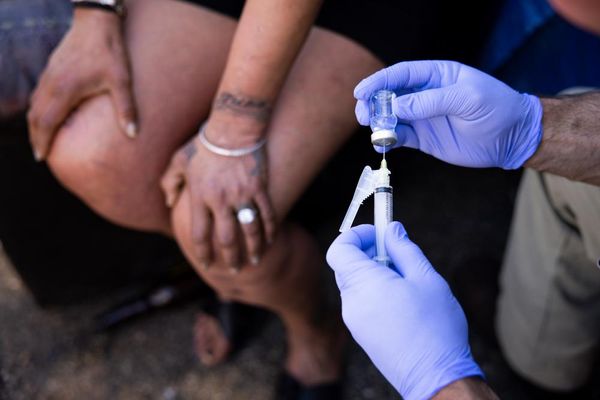
Although mainly known for its anaesthetic properties, ketamine can be used as a psychedelic if administered in lower doses, where it triggers hallucinations and causes a state where individuals feel distant from reality, hence its classification as a dissociative drug.
Several research groups conducted studies and gathered evidence underlying the potential of ketamine in mental health therapeutics, especially for depression and alcoholism. It is crucial to highlight that the administration of the psychedelic substance on its own (for ketamine as for other psychedelics) should be coupled with psychotherapy for the treatment to be safer and more effective. Hence the term “psychedelic-assisted therapy” (“ketamine psychedelic therapy” or “KPT” in this case) which has already been studied and applied in some countries during the last century before the widespread ban of psychedelics.
Generally, therapy sessions with a psychiatrist take place days before and after the administration of the drug (“preparation” and “integration”, respectively), whereas during the psychedelic trip a clinician and in some cases a guide are present to monitor and support the patient throughout the trip. In a 10-year study where AUD patients had intravenous injections of ketamine, they could smell alcohol during the session, to associate negative feelings that occurred during the psychedelic trip with the addictive substance.
The safety and brevity of a ketamine trip make this substance more advantageous compared to other psychedelics, not to mention the results of the studies conducted so far, where patients described their experience as “cathartic” and “resolutive”, and showed positive changes in their way of relating to life, themselves, and the world.
With all this considered, no wonder there’s growing enthusiasm for introducing ketamine as a treatment for alcohol addiction.
Awakn’s phase 2b trial
Concluded in 2021, Awakn’s (OTC:AWKNF) KARE (Ketamine for Reduction Alcoholic Relapse) phase 2b trial was led by Profession Celia Morgan from the University of Exeter in collaboration with University College London and the Imperial College. The double-blind trial involved the participation of nearly 100 people affected by severe alcoholism, who used to drink daily before the start of the trial (125 units per week).
The clinical study had two aims; firstly, to compare ketamine with placebo in terms of its safety and efficacy in increasing alcohol abstinence in individuals affected by severe alcohol use disorder. Secondly, to assess something which has never been assessed in any mental health research study involving ketamine (until now): the effects of ketamine administration with vs without therapy (including some alcohol education).
Therefore, the patients were randomly allocated to 4 different groups: 1) ketamine + therapy 2) placebo + therapy 3) ketamine with no therapy 4) placebo with no therapy). All receiving 3 infusions per week, with progress being assessed through behavioural and psychological tests 3 and 6 months after the treatment.
Remarkably, the ketamine + therapy group was found to reach almost 90% abstinence in the 6 months follow-up, which was more than twice as much as the placebo group; and for some people, relapse was prevented even over the 6 months. Improvements were also observed in decreased depression symptoms 3 months after the treatment in both ketamine groups (with and without therapy). After ketamine + therapy, participants just drank 5 days over the total 6 months follow-up, which comports a significant reduction in the risk of alcohol-related death.
The next steps: phase III trial
Considering these results, it was not surprising that Awakn announced an upcoming phase III trial. Costing roughly $3.75 million, phase III will receive 66% of the funding from the UK government agency NIHR (National Institute for Health and Care Research), with the rest covered by Awakn. In this case, 280 patients are meant to take part in the study and will be followed over 6 to 12 months after the treatment, where peer support groups will probably be included as well as a post-treatment aid.
The whole study is part of Project Kestrel, Awakn’s clinical development program, aiming to get the approval of ketamine-assisted therapy for alcohol use disorder in the UK and the US, a goal that will potentially help countless numbers of patients.