There’s an entire world of microbes living in our gut and typically, that’s a very good thing. A healthy gut microbiome is increasingly associated with health in other areas of the body, often by keeping inflammation in check. But there’s also a perpetual battle happening between “good” microbes — those are the ones that help us maintain optimal health — and “bad” microbes, which can contribute to inflammation and other health issues.
Some bacteria found in the gut can contribute to Inflammatory Bowel Disease. But using antibiotics to eradicate these bacteria can do more harm than good. Antibiotics aren’t precise, and when they roll through the gut they can wipe out much of the beneficial bacteria along with any harmful bacteria. On top of that, antibiotics can trigger antibiotic-resistant bacteria — organisms that evolve to evade the antibiotics’ powers.
That’s why researchers in Israel are taking a different approach — one that harnesses the power of bacteria-killing viruses called bacteriophages. Through that research, scientists may have discovered an innovative treatment for IBD. The researchers’ study, published today in the journal Cell, outlines how they made the discovery.
The background— Inflammatory Bowel Disease affects millions of people worldwide; the Centers for Disease Control estimates that in the United States alone, 3.1 million people have been diagnosed with IBD. People who have Crohn’s Disease and ulcerative colitis fall under the umbrella of IBD.
It’s a condition Eran Elinav, a microbiome researcher and professor at the Weizmann Institute of Science in Israel and the German National Cancer Center, has been studying with his colleagues for “many years,” he tells Inverse. While that research has led to “lots of insights on the combination of genetic susceptibility and environmental factors that drive it, treatment remains disappointing and non-curative,” he says.
“As part of our IBD research, we know that gut microbes are exceedingly important in contributing to IBD, but we’ve so far failed to comprehensively identify them at the strain level.”
Elinav and his colleagues set out to do two things: identify the exact bacterial strains that play a role in human intestinal inflammation and develop a “precision weapon” that would “selectively and effectively treat” disease-causing organisms in the microbiome, without harming any of the beneficial microbes in the gut. This kind of “weapon” could dramatically improve the lives of millions of people with IBD.
The hypothesis — Elinav and his team have long thought that viruses called phages — or bacteriophages — could be a promising weapon in the fight against disease-causing bacteria. Phages are the most abundant organisms on Earth, they’re found wherever bacteria are found, and thus flourish in the human gut. Since early in the 20th century, scientists have tried to use phages to treat infectious diseases, but the ease of antibiotics stalled many of those efforts. Yet phages can do what antibiotics can’t: Target and eliminate specific bacteria while leaving the rest of the microbiome untouched.
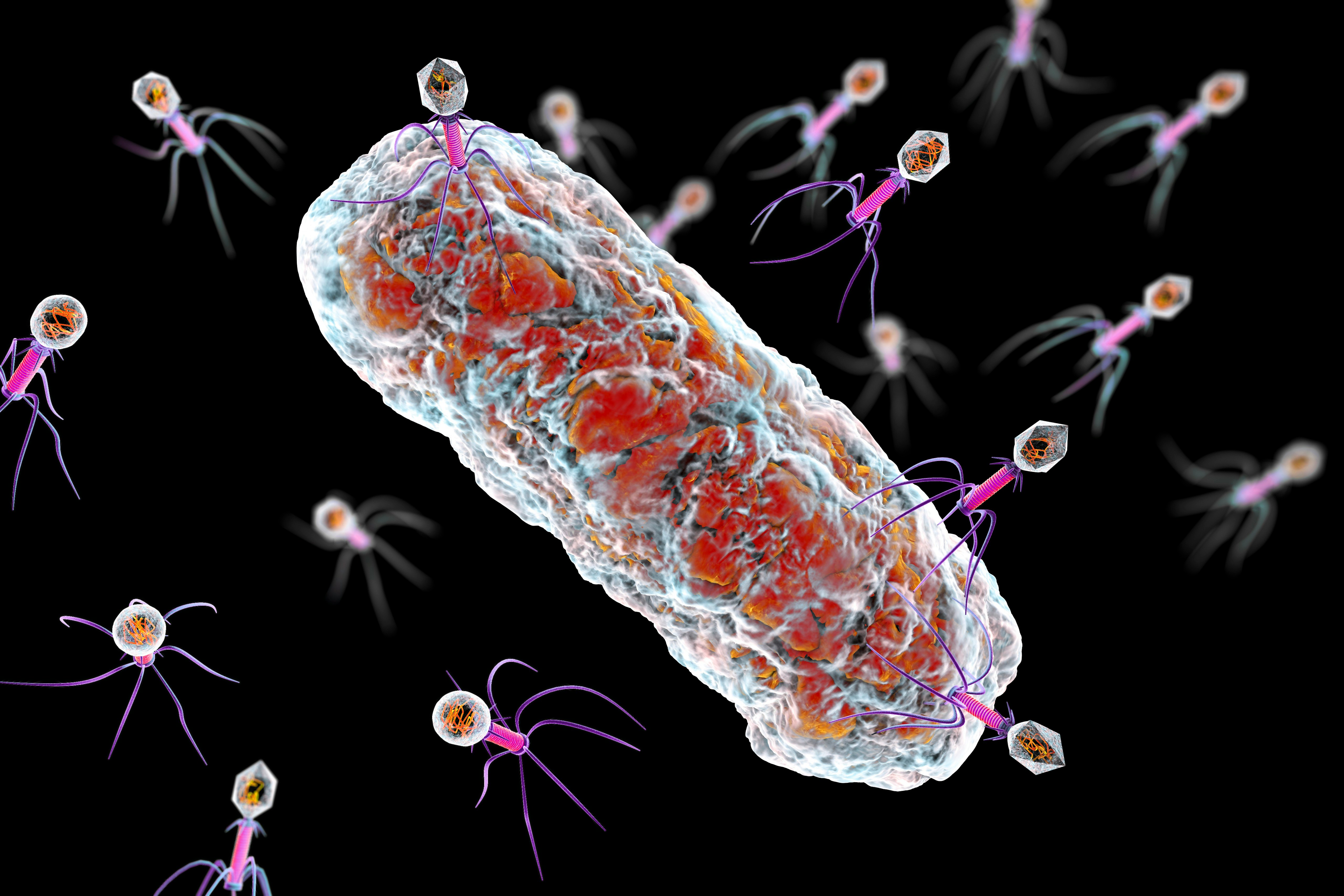
It’s promising in theory, but not quite as simple as it may sound. Elinav explains, “there is a constant ‘arms race’ between bacteria and their major enemies, phages, and bacteria have developed very effective mechanisms to resist phage infection, such as the recently discovered CRISPR system,” he says.
“Thus, applying a single phage will universally result in resistance developing in target bacteria that will render it ineffective.”
An additional challenge is that administering these phages can sometimes result in an immune response against the phage itself. After all, our immune systems are designed to recognize and neutralize foreign pathogens, which is exactly what these phages are.
How the discovery was made — Before figuring out how to harness phages’ power to neutralize disease-causing bacteria, the researchers needed to determine exactly which bacteria they should target.
“On the one hand, there is an enormous and constantly expanding realization that the gut microbes play critical roles in IBD development, progression, complications, and even responses to treatment,” Elinav says. On the other hand, the researchers hadn’t been unable to identify the specific disease-causing microbes or develop a treatment against them.
They started by comparing the composition of gut microbes in healthy volunteers with the gut microbes of people with ulcerative colitis and Crohn’s disease, two major forms of Inflammatory Bowel Disease. Study participants included people from France, Germany, Israel, and the United States.
A computational analysis of the difference in gut microbes between the people with and without IBD allowed the researchers to zero in on several bacterial strains that were plentiful in people with IBD, but absent in the microbes taken from people in the control group.
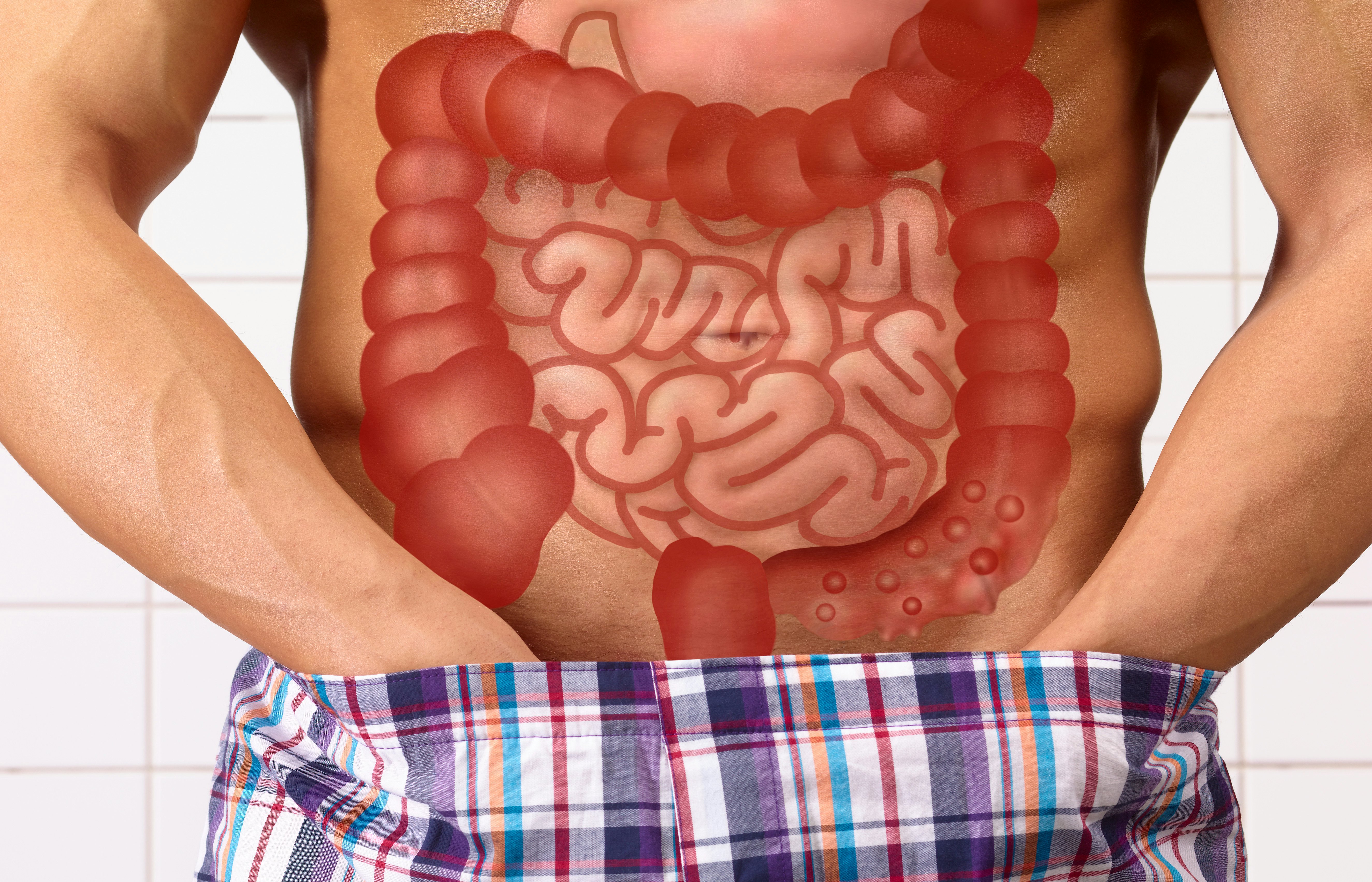
What they found — Multiple strains of the Klebsiella pneumoniae bacterium were present in patients with IBD, but not the other volunteers. To test their hypothesis that these strains contributed to IBD, the researchers implanted the bacteria into mice. Sure enough, the mice experienced inflammation and intestinal damage.
Now that the researchers had their target, they pivoted to developing a precision phage weapon.
“We then isolated thousands of phages from samples collected from sewers, dental waste, and microbiome samples,” Elinav says. “[We] grew and characterized them, and engaged in a prolonged iterative ‘mix and match’ experiments designed to identify the optimal combination of phages that would target the IBD-associated Kp [Klebsiella pneumoniae] strains.”
The researchers wanted to develop a “cocktail” of phages that could attack and kill the bacteria through a variety of different mechanisms in the hopes that such a mix would thwart the bacteria’s ability to develop resistance against all the phages.
“We ended up with a ‘winner’ 5-phage cocktail that effectively suppressed the IBD-linked Kp strains both in the test tube and in mice,” Elinav says. “We’ve tested the 5-phage cocktail in animal models of IBD and demonstrated that they were able to reach the gut, suppress Kp, and ameliorate the inflammation and tissue damage that it inflicts in the IBD setting.”
In a subsequent experiment, the researchers gave two representative phages in the cocktail to study volunteers who did not have IBD to see if the therapy was safe and well tolerated.
“We were able to show that, when orally taken with antacids, phage remain viable and accumulate in the human gut in concentrations exceeding those expected to kill Kp by nearly a thousand-fold,” Elinav says. “Phage therapy was safely tolerated and had minimal impacts on the surrounding human microbiome.”
What’s next— The researchers are now expanding on these discoveries to begin a Phase II trial that will test the cocktail’s effectiveness in IBD patients. They also hope to develop “phage cocktails targeting other disease-causing gut microbes including metabolic, inflammatory, neoplastic and even neurodegenerative disease,” Elinav says.
Indeed, the potential applications for this kind of precision medicine are boundless.