Compared to men, we know much less about how women experience disease.
Biomedical research helps us understand the timeline of diseases and how we can treat them. In the past, most of it has been conducted on male cells and experimental animals, such as mice. It has been assumed the results from such “pre-clinical” research on males apply to females too.
Yet men and women experience disease differently. That includes how diseases develop, the length and severity of symptoms, and the effectiveness of treatment options.
Smaller bodies?
Although these differences are now widely acknowledged, they are not fully understood. And women are often worse off as a result.
This is the case for prescription drugs. Women experience around 50-75% more adverse reactions than men. This results in many drugs being pulled from the market due to concerns over health risks for women.
Drug reactions in women have been argued to be due to sex differences in body weight rather than differences in how the drug works in the body.
Therefore, it’s thought that if drug doses are adjusted according to body weight, women will often receive lower doses than they do now – which may alleviate adverse reactions.
But that may not be the case.
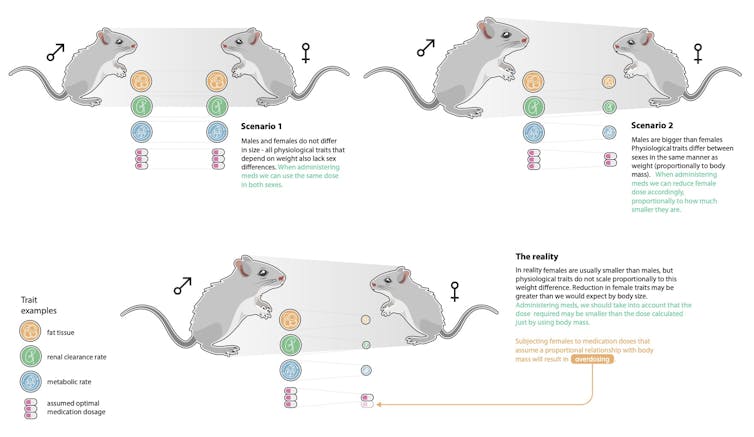
In new research published today in Nature Communications, we show this basic assumption in biomedicine – that females are “smaller versions” of males – is not supported for most pre-clinical traits (things like glucose levels, for example).
So, drug reactions in women are unlikely to be alleviated simply by adjusting the dose to one’s body weight.
Adverse drug reactions are common and costly for healthcare
Basing women’s healthcare decisions based on research conducted on men – and vice versa – has potentially profound consequences. In the case of adverse drug reactions, the impacts are significant from both a clinical and economic perspective.
A recent study estimated that 250,000 hospital admissions in Australia each year are medication related, costing the healthcare system around $1.4 billion annually.
Read more: As pharmaceutical use continues to rise, side effects are becoming a costly health issue
Drug reactions have also been shown to lengthen hospital stays. In a large UK study, patients admitted to hospital with an adverse drug reaction stayed for a median of eight days.
Women often cite adverse reactions as the reason for discontinuing medications. If weight-adjusted dosing of drugs could reduce adverse drug reactions, we would see women receive greater potential benefit from the healthcare system.
The weight of evidence
But what evidence do we have that weight adjustment will work? The US Food and Drug Administration (FDA) has already recommended dosage changes for women for some drugs (such as the sleep drug zolpidem). Additionally, weight-adjusted dosing for some antifungal drugs and antihypertensive drugs appears to work.
On the other hand, drug reactions are strongly linked to what the drug does in the body in women , and less so in men. There are also many documented differences in physiology between men and women that relate to how drugs are absorbed and cleared by the body, and not to body weight.
To get to the bottom of this, a broad scale approach is needed. We borrowed a method routinely used in evolutionary biology, known as “allometry”, where a relationship between a trait of interest and body size is examined on a log scale.
We applied allometry analyses to 363 pre-clinical traits in males and females, comprising over two million data points from the International Mouse Phenotyping Consortium.
We focused on one of the most common disease model animals: mice. We asked whether sex differences in pre-clinical traits – such as fat mass, glucose, LDL cholesterol - could be explained by body weight alone.
Read more: Got high cholesterol? Here are five foods to eat and avoid
Our analyses recovered sex differences in many traits that cannot be explained by body weight differences. Some examples are physiology traits, such as iron levels and body temperature, morphology traits such as lean mass and fat mass, and heart traits such as heart rate variability.
We found the relationship between a trait and body weight varied considerably across all the traits we examined, meaning that the differences between males and females could not be generalized: females weren’t simply smaller versions of males.
Ignoring these differences in some cases, such as measures of blood cells, bone and organs, could result in missing a lot of the population variation for a particular trait: up to 32% for females and 46% for males.
This complexity means we need to consider sex differences for drug dosing on a case-by-case basis.
One size does not fit all
In an era where personalised medicine interventions are within reach, and patient-specific solutions are on the horizon, we now know that sex-based data are much needed to advance care in an equitable and effective manner.
Our study uncovers the ways in which males and females can vary across many pre-clinical traits, indicating that biomedical research needs to focus more closely on measuring how and in what ways the sexes differ.
Particularly, when a relationship between sex and drug dose is uncovered, our data suggest dose-response is likely to be different for males and females.
The methods in our study could help clarify the nature of these differences and provide a path forward to reducing drug reactions.
Read more: The evolutionary history of men and women should not prevent us from seeking gender equality
Laura A. B. Wilson receives funding from the Australian Research Council.
Shinichi Nakagawa receives funding from the Australian Research Council.
This article was originally published on The Conversation. Read the original article.







