
As the population ages, the number of people with neurodegenerative diseases, such as Alzheimer’s disease, increases. Approximately 75,000 Canadians are diagnosed with Alzheimer’s disease each year and experience a decline in their cognitive abilities. The ordeal usually lasts for several years while their family members watch helplessly.
Neurodegenerative diseases are characterized by proteinopathies — abnormal accumulations of proteins in the brain that impair the functioning of neurons. The most widely studied therapeutic approach to developing drugs for Alzheimer’s is to try to reduce the aggregation of amyloid-beta peptide and tau protein in neurons.
However, in order to reach their targets, the drugs must first cross the blood-brain barrier (BBB) from the blood to the brain. This is because endothelial cells, cells that line the tiniest blood vessels in the brain, regulate the exchange between blood and the brain. They maintain a balance that allows access to essential molecules such as glucose, but restrict the passage of most pharmaceuticals, including the new and much-hyped drug lecanemab.
When these brain endothelial cells become diseased, the balance is upset. The brain struggles to get the substances it needs back into the circulation and rejects those that might harm it.
The brain and the other organs of the body are thus in constant communication, while in health or in disease.
As experts in neurodegenerative diseases and the BBB, we have conducted a study on insulin receptor dysfunction in Alzheimer’s disease.
Insulin and the brain
Insulin is an essential hormone for life. It is best known for its effect on the regulation of blood sugar and remains an essential part of the pharmaceutical treatment of diabetes. In recent decades, researchers have noted vascular and metabolic abnormalities in a high proportion of patients with dementia.
Indeed, Type 2 diabetes, characterized in the later stages by insulin resistance, is a major risk factor for Alzheimer’s disease. There is some evidence to suggest that the Alzheimer’s brain is less responsive to insulin. Conversely, studies have shown that insulin can improve memory, prompting the development of clinical trials on the effect of insulin on Alzheimer’s disease.
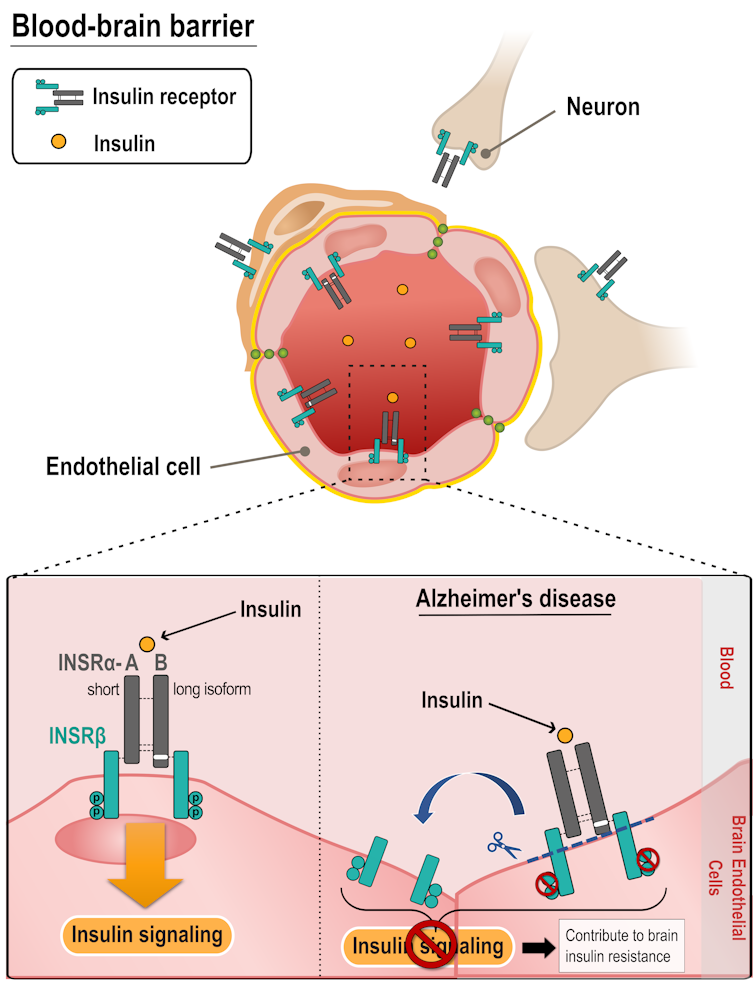
Yet we still don’t know what cell types and mechanisms are involved in the action — and loss of action — of insulin in the brain. The vast majority of insulin is produced by the pancreas and secreted into the bloodstream. Therefore, to affect the brain, insulin must first interact with the BBB and its endothelial cells, which are in contact with the blood and can take up insulin through receptors.
Alzheimer’s and the insulin receptor
In order to measure the amount of these insulin receptors in the brain, we performed analyses directly in human tissues. These samples came from a cohort of over a thousand people who agreed to donate their brains after death. We have access to them through a partnership with researchers at Rush University in Chicago.
We found that the insulin-binding receptor is predominantly located in the microvessels, so, within the BBB itself. Moreover, the abundance of this receptor is decreased in Alzheimer’s patients. This decrease could lead to the loss of insulin response in the Alzheimer brain.
Insulin receptor dysfunction
In order to better control the experimental variables and measure the response of the insulin receptor, we then tested our hypotheses in mice. The in situ cerebral perfusion technique consists of injecting insulin directly into the carotid artery (an artery located in the neck) so that it reaches the brain in its entirety. We have shown that circulating insulin mainly activates receptors located on the cerebral microvessels.
Although it was generally accepted that insulin crosses the BBB to reach cells such as neurons deeper in the brain tissue, our results show that the proportion of insulin that crosses the BBB is low.
These two observations thus confirm that the majority of insulin must interact with cells in the BBB before it can exert an action on the brain.
We then applied the same method to transgenic mice, which were genetically modified to model Alzheimer’s disease. We found that the response to insulin at the BBB was dysfunctional, with no activation of the insulin receptor in these diseased mice.
Thus, in both humans and rodents, the brain insulin receptor is located primarily at the BBB, and its ability to respond to blood insulin is impaired in Alzheimer’s disease.
A significant breakthrough
In sum, our results suggest that alterations in the number, structure and function of insulin receptors at the level of BBB endothelial cells may contribute to the cerebral insulin resistance observed in Alzheimer’s disease.
Alzheimer’s research efforts are currently focused on drugs that, in order to reach their therapeutic target, the neurons, must first cross the BBB, which severely restricts their passage. By targeting the metabolic dysfunction of the brain instead, we propose a research alternative that has two major advantages.
The first is that we can use treatments that do not have to cross the BBB barrier, since it is the endothelial cells themselves that become the therapeutic target. The second involves “drug repurposing,” which consists of taking advantage of the phenomenal therapeutic arsenal already approved to fight diabetes and obesity, but using this in the context of Alzheimer’s.
It should be remembered that the few drugs available to us provide only a modest improvement in symptoms. Combating insulin resistance in the brain would make it possible to break the vicious circle between neuropathology (disease that affects the brain) and diabetes, and in theory slow down the progression of the disease.
The work is not finished
On the basic research side, we will continue to study the mechanisms downstream from the microvessels to understand the action of insulin on the deep layers of the brain.
We hope that clinical research will follow suit with human studies to repurpose drugs that target certain metabolic diseases, such as diabetes, towards fighting Alzheimer’s.
In the meantime, while waiting for pharmaceutical solutions, each of us would do well to adopt the preventive cocktail that we all know well: a healthy diet combined with frequent physical and mental exercise.
Frederic Calon has received funding from: Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Fonds de la recherche du Québec en santé (FRQS), Alzheimer Society of Canada.
Manon Leclerc has received scholarships from the Fondation du CHU de Québec and the Fonds de Recherche du Québec - Santé (FRQS).
Vincent Emond does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
This article was originally published on The Conversation. Read the original article.







