Your support helps us to tell the story
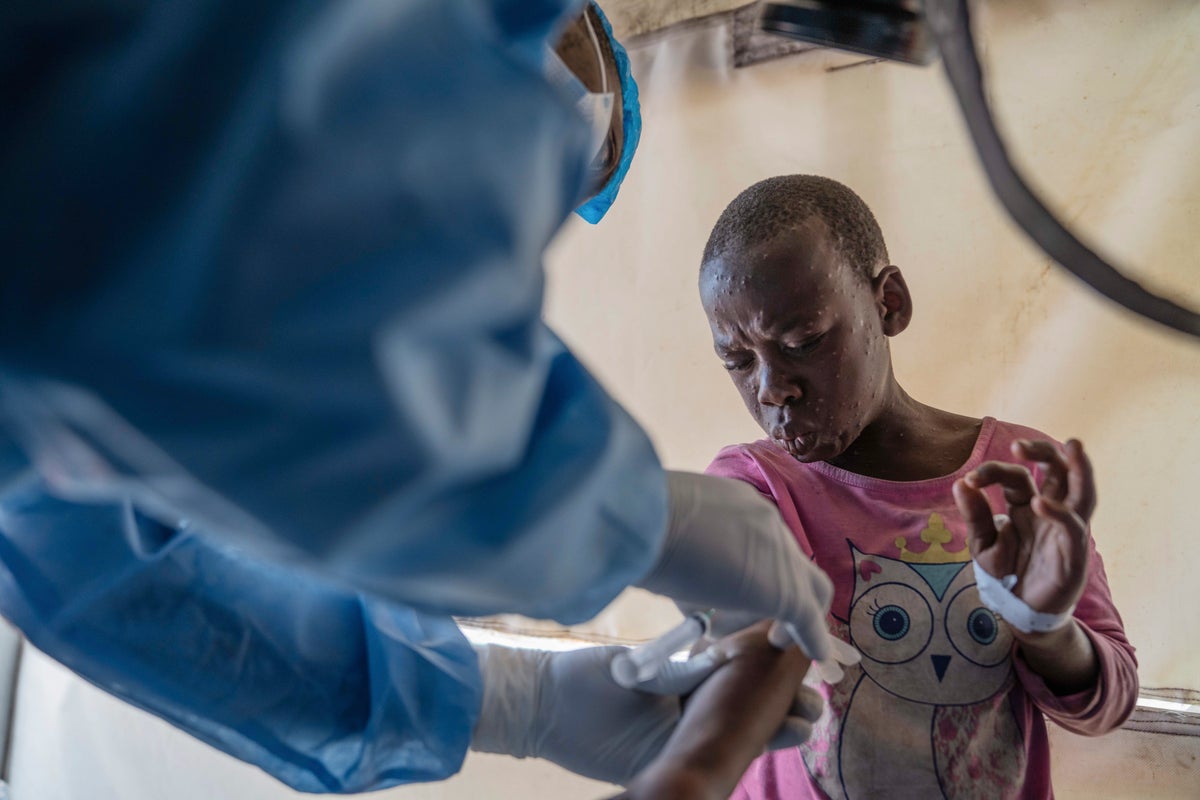
A vaccination campaign against mpox in Congo will begin Oct. 2, authorities said Saturday, with workers focusing on the three most affected provinces first.
Adults in Equateur, South Kivu and Sankuru provinces will be vaccinated first, Cris Kacita Osako, coordinator of Congo’s Monkeypox Response Committee, told The Associated Press.
Earlier this week, the first batch of mpox vaccines arrived in the capital of Congo, the center of the outbreak. The 100,000 doses of the JYNNEOS vaccine, manufactured by the Danish company Bavarian Nordic, were donated by the European Union through HERA, the bloc’s agency for health emergencies. Another 100,000 were delivered on Saturday.
The 200,000 doses are just a fraction of the 3 million doses authorities have said are needed to end the mpox outbreaks in Congo, the epicenter of the global health emergency. The European Union countries pledged to donate more than 500,000 others, but the timeline for their delivery remained unclear.
Since the start of 2024, there have been 5,549 confirmed mpox cases across the continent, with 643 associated deaths, representing a sharp escalation in both infections and fatalities compared to previous years. The cases in Congo constituted 91% of the total number. Most mpox infections in Congo and Burundi, the second most affected country, are in children under age 15.
On Friday, the Africa Center for Disease Control and Prevention and the World Health Organization launched a continent-wide response plan to the outbreak of mpox, three weeks after WHO declared outbreaks in 12 African countries a global emergency.
Congo issued an emergency approval of the vaccine, which has already been used in Europe and the United States in adults. For the moment, the rollout will be reserved for adults, with priority targeted groups being those who have been in close contact with infected people and sex workers, the Africa Center for Disease Control and Prevention Director-General Dr. Jean Kaseya told reporters on Friday.
The European Medicines Agency is examining additional data to be able to administer it to children ranging in age from 12 to 17, which could happen at the end of the month, HERA Director-General Laurent Muschel said.







