Today’s gene therapies have huge potential — they have worked in treating certain cancers, along with blood and vision diseases — but they can come with major downsides. For example, they’re super pricey to develop, can be time-consuming, and may bring significant side effects like inflammation, organ damage, and even cancer.
Gene therapies work by manipulating the genes that cause or lead to certain diseases and restore cells’ normal functioning. Scientists often accomplish this by engineering viruses or tiny substances called nanoparticles to carry genes or gene-editing tools directly to cells.
But many things can go wrong in this process. One main issue is that these gene therapy vehicles can travel to the wrong places and damage healthy cells. Now, scientists think that a type of virus with an appetite solely for bacteria — known as a bacteriophage — could overcome these obstacles.
One type of bacteriophage, called T4, is one of nature’s most efficient viruses and could offer a cheaper, safer, and more efficient gene therapy technique, according to a new study published in the journal Nature Communications.
“The safety and technological leap that this will have is very significant,” Venigalla Rao, a biochemist at the Catholic University of America and co-author of the new study, tells Inverse. Rao has studied bacteriophage T4 for over two decades — and now the mighty virus may finally see the limelight.
A vehicle upgrade
Scientists have been engineering viruses for therapeutic uses since the 1960s, including for the development of vaccines. Doctors typically give patients the gene therapy vehicle, referred to as a vector, by injection or through an IV. Common vectors include small viruses called adeno-associated viruses, which already infect humans but haven’t been found to cause diseases, and lentiviruses, which include the virus that causes HIV but is engineered to be safe for patients.
A newer technique harnesses nanoparticles, which can be more easily produced in the lab and are less likely to cause harmful side effects. But the FDA hasn’t yet approved any therapies that use nanoparticles because they still come with technical issues, such as their tendency to degrade in the human body and questions over their long-term safety.
If it succeeds in trials, bacteriophage T4 could offer some distinct advantages over existing gene therapy methods, Rao says. It can be engineered in a test tube, where it is easily customized — meanwhile, most virus vectors must be grown in large amounts of cells, which can be time-consuming and expensive. Within about two to three hours, Rao notes he can grow 100 trillion bacteriophage particles — pharmaceutical companies could accomplish this even faster with more complex equipment.
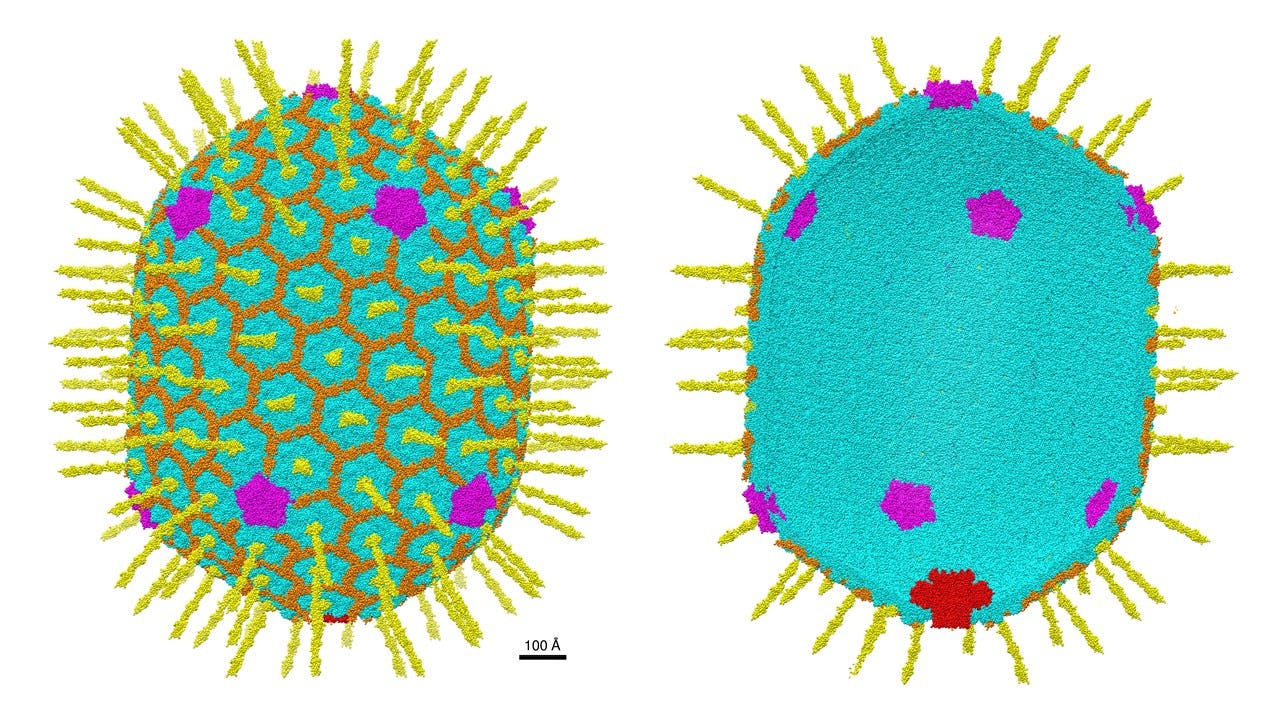
Rao and his team can “mix and match” many types of molecules into bacteriophage T4 based on the specific condition being targeted in each patient and their genetic makeup. Plus, T4 is relatively roomy and versatile compared to today’s options. “The engineering capability is superior,” he says.
Bacteriophage T4 therapy could therefore be engineered to benefit people with rare conditions, who often must seek out very expensive therapies — or may not have any treatment options at all.
Bacteriophage T4 may also prove safer than current gene therapy options. Due to its highly customizable structure, it could reduce the risk of including extra genetic material and damaging healthy surrounding cells — many virus- and nanoparticle-based delivery systems still struggle with this issue.
Rao and his colleagues haven’t yet performed safety trials in people, but he points to the fact that bacteriophages already live in our gut, where they seem to benefit our overall health.
At the moment, he’s focused on creating therapies with T4 for muscular dystrophy and sickle cell disease. How long it will take to reach the market largely depends on funding. Rao’s team is currently working with human cells in the lab, but they would like to move on to animal models and eventually run human trials.
While this work remains in the early stages, he thinks the new study could make waves in the research community and even inspire other teams to fast-track similar bacteriophage technologies. But for now, he’s confident in the potential of his innovative new method.
“We have optimized this technology, and we have a pretty good chance of going toward therapies and potentially cures in the future much more rapidly than with any other bacteriophage,” he says.







