
Stem cells are the starting point for all other cells in our bodies. The first such cells to be found were blood stem cells – as the name suggests, they give rise to different types of blood cells.
But there’s much we don’t know about how these cells develop in the first place. In a study published today in Cell Reports, we have shown how a lab simulation of an embryo’s beating heart and circulation lead to the development of human blood stem cell precursors.
The tiny device mimics embryonic blood flow, allowing us to directly observe human embryonic blood formation under the microscope. These results may help us understand how we can produce life-saving therapies for patients who need new blood stem cells.
Growing life-saving therapies in the lab
To treat aggressive blood cancers such as leukaemia, patients often need extremely high doses of chemotherapy; a blood stem cell transplant then regenerates blood after the treatment. These are life-saving therapies but are restricted to patients who have a suitable tissue-matched donor of blood stem cells.
A way around this problem would be to grow more blood stem cells in the lab. Unfortunately, past experiments have shown that harvested adult blood stem cells lose their transplantation potential if grown in the lab.
The discovery of induced pluripotent stem cells – stem cells made out of adult cells – in 2006 led to a promising new approach. Induced pluripotent stem cells are made from the patient’s own cells, so there is no problem with tissue rejection, or the ethical issues surrounding the use of IVF embryos.
These cell lines are similar to embryonic stem cells, so they have the potential to form any tissue or cell type – hence, they are “pluripotent”. In theory, pluripotent stem cell lines could provide an unlimited supply of cells for blood regeneration because they are immortalised – they can grow in the lab indefinitely.
But the development of processes to allow us to grow particular types of tissues, organs and cell types – such as blood – has been slow and will take decades to advance. One must mimic the complex process of embryogenesis in the dish!
Read more: World's first 'synthetic embryo': why this research is more important than you think
Engineering an embryonic heart
Current understanding of how embryonic blood stem cells develop is based on animal models. Experiments with anaesthetised zebrafish embryos have shown that blood stem cells arise in the wall of the main blood vessel, the aorta, shortly after the first heartbeat. For ethical reasons, it’s obvious this type of study is not possible in human embryos.
This is why we wanted to engineer an embryonic heart model in the lab. To achieve this, we used microfluidics – an approach that involves manipulating extremely small volumes of liquids.
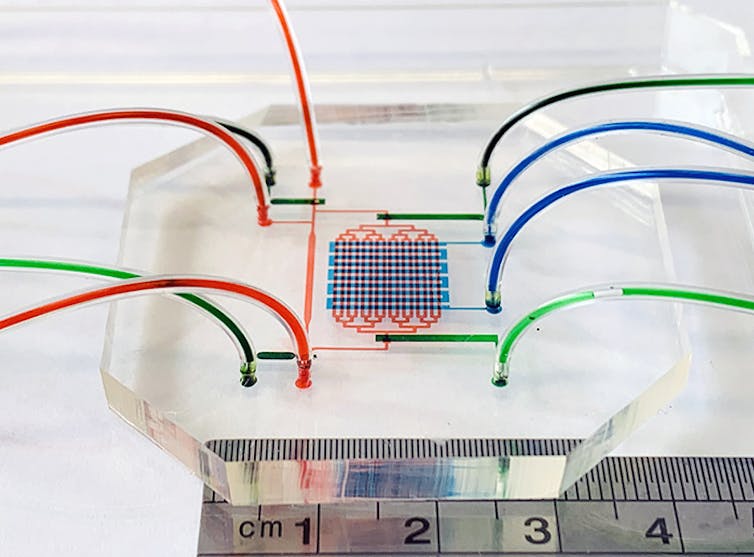
The first step in generating blood stem cells from pluripotent stem cells is to coax the latter to form the site where blood stem cells start growing. This is known as the AGM region (aorta-gonad-mesonephros) of the embryo.
Our miniature heart pump and circulation (3 by 3 centimetres) mimics the mechanical environment in which blood stem cells form in the human embryo. The device pumps culture media – liquids used to grow cells – around a microfluidic circuit to copy what the embryo heart does.
A step closer to treatment
Once we got the cells to form the AGM region by stimulating cells on day two of starting our cell culture, we applied what’s known as pulsatile circulatory flow from day 10 to day 26. Blood precursors entered the artificial circulation from blood vessels lining the microfluidic channels.
Then, we harvested the circulating cells and grew them in culture, showing that they developed into various blood components – white blood cells, red blood cells, platelets, and others. In-depth analysis of gene expression in single cells showed that circulatory flow generated aortic and blood stem precursor cells found in the AGM of human embryos.
This means our study has shown how pulsatile circulatory flow enhances the formation of blood stem cell precursors from pluripotent stem cells. It’s knowledge we can use in the future.
The next step in our research is to scale up the production of blood stem cell precursors, and to test their transplant potential in immune-deficient mice that can accept human transplants. We can do this by using large numbers of pluripotent stem cells grown in bioreactors that also mechanically stimulate blood stem cell formation.
If we can easily produce blood stem cells from pluripotent stem cell lines, it would provide a plentiful supply of these cells to help treatments of cancer or genetic blood diseases.
This research was funded by Stem Cells Australia (ARC Special Research Initiative in Stem Cell Science) and an internal grant from the Faculty of Engineering, University of New South Wales.
Jingjing Li received Australia Postgraduate Award (APA) scholarship, Women in Engineering Research Top-Up Award and Engineering Supplementary Award (ESA) from University of New South Wales.
This article was originally published on The Conversation. Read the original article.







