
Scotland and Wales are going to offer Covid-19 jabs to children aged five to 11.
Here are the answers to some of the key questions about vaccines for younger children.
– What is the latest?
Ministers in Wales and Scotland have said they have seen guidance from the Joint Committee on Vaccination and Immunisation (JCVI) which advises that children in this age group should be offered the vaccine.
As a result, they have decided to press ahead with offering Covid-19 jabs to children aged five to 11.
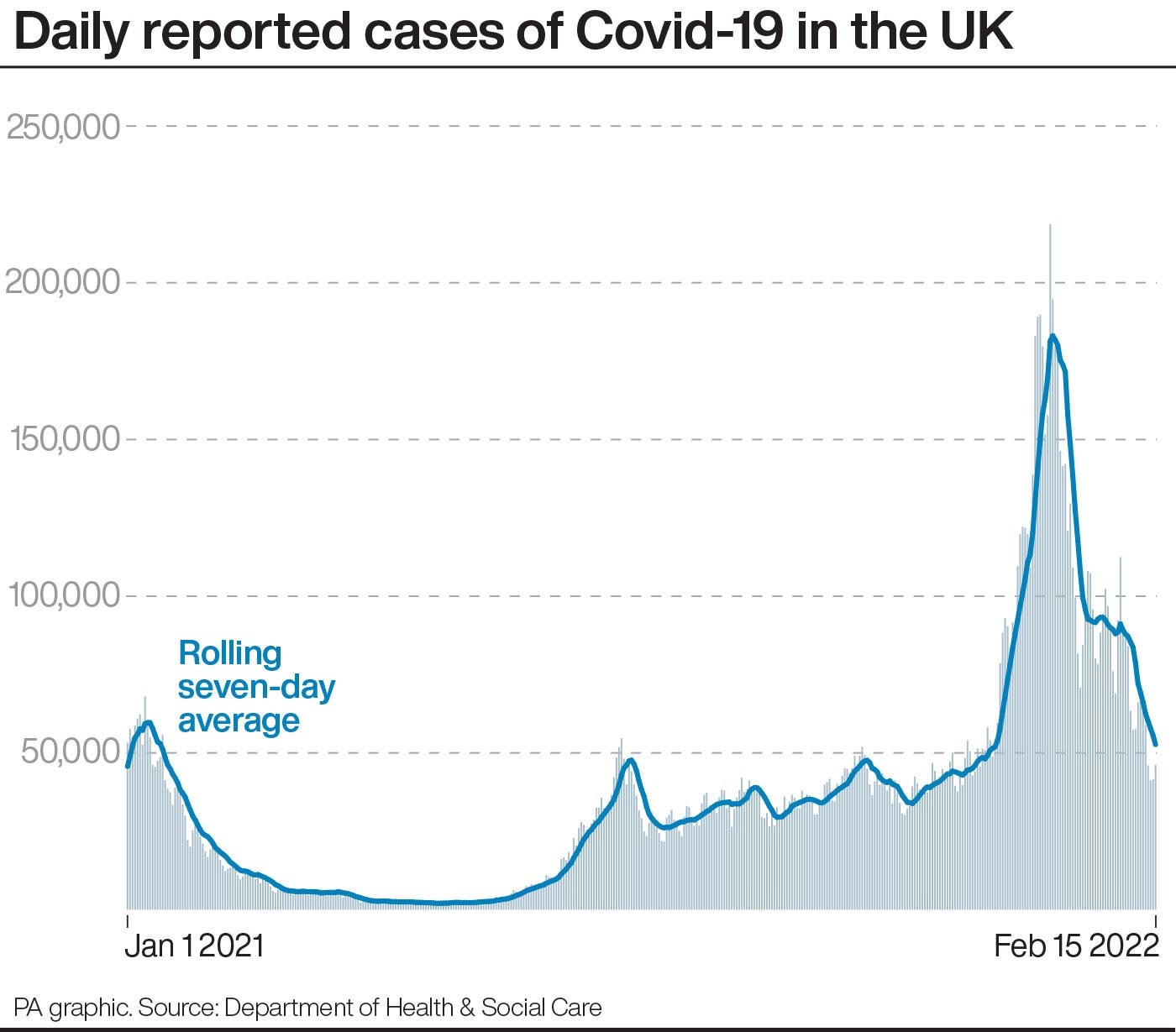
The JCVI guidance is yet to officially be published but is expected soon.
– Did jabs not get approved for use in five to 11-year-olds in December?
The usual protocol for vaccine approval starts with the UK medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), which confirms whether or not a vaccine is safe and effective after looking at all of the data submitted by pharmaceutical companies.
Vaccination experts on the JCVI then assess this data and other factors – in this case school absences and rates of infection – before they advise ministers as to whether they should offer a vaccine.
Ministers then announce their decision.
The MHRA announced in December that a special “paediatric formulation” of the Pfizer/BioNtech Covid-19 vaccine was safe for use among children aged five to 11.
The JCVI then said that the vaccine could be offered to “at risk” children in this age group, but reserved its decision on wider use among this cohort. Its official announcement is expected later on Wednesday.
– What is going on with the disparities across the UK?
In this case, it appears that the JCVI guidance has been given to ministers but not yet been announced.
A bit of political wrangling in Westminster could be to blame and it may be that Whitehall officials wanted to announce the decision on offering jabs for five to 11-year-olds when then set out their Living With Covid plans next week.
Now Wales and Scotland have jumped the gun on the announcement, it has forced the hand of Westminster officials and the JCVI decision is expected to be published on Wednesday.
Announcements from ministers in England and Northern Ireland will follow.
– Can you tell me more about this “paediatric formulation”?
Children aged five to 11 will be offered a much lower dose of the vaccine – lower than that offered to adults or children aged 12 and over.
They will be offered a 10 micrograms dose compared with 30 micrograms.
The MHRA said the vaccine is given as two injections in the upper arm. It will be for the JCVI to make the final recommendation on the so-called dosing interval – or how far apart the vaccines should take place.
– Are there any side effects?
When the MHRA approved the jab for younger children is said that its review of side effects found that the “overwhelming majority” were mild, including a sore arm and flu-like illness.

It examined lots of data, including information from the US where millions of children aged five to 11 have been vaccinated. The jab has also been on offer to this age group in a number of countries around the world.
– Where will children be given jabs?
It has not yet been announced where children will be vaccinated.
Children aged 12 and over and vulnerable five to 11-year-olds have been able to use NHS facilities and some schools have been used as vaccination sites.
– How many children will be offered the jab?
There are an estimated 5.8 million five to 11-year-olds in the UK.
Of course some of these will have already been offered a chance to have a vaccine if they are in vulnerable groups.
– Will children under the age of five ever be offered the jab?
It is a possibility but those decisions are a long way off.
The UK has taken time to weigh up the risks and benefits of vaccinations for children and often taken much longer than some other western countries to confirm it is happy for its youngest citizens to get a Covid jab.
Last week the US Food and Drug Administration (FDA) said it needed more data before considering whether children aged six months to four years should be offered the Pfizer vaccine.





