
For as long as humans have grown our own food, we have battled pest animals that destroy crops and take food for themselves.
The traditional approach has been to try to kill the pests, typically with poisons. Too often, however, this fails to kill enough pests, harms native animals, and only minimally reduces damage.
We tackled this problem in a different way by asking: how do we stop hungry animals finding our crops in the first place?
In a research paper published today, we show how “chemical camouflage” can prevent house mice finding newly sown wheat seeds. The method reduced mouse damage to wheat crops by more than 60% even during plague conditions, without killing a single mouse.
The rodent menace
Rodents are responsible for an estimated 70 million tonnes of grain lost worldwide each year. Even a 5% reduction in these losses could feed more than 280 million people.
In Australia, the 2021 mouse plague cost farmers in New South Wales alone upwards of $1 billion, according to an industry association estimate. A mouse plague occurs somewhere in Australia at least every four years.
Currently, the only management option to reduce mouse numbers is broad-scale baiting. However, baiting is often ineffective and has led to calls for more lethal poisons, which carry major risks for native wildlife.
The relationship between baiting effort and crop yield is not well understood, and mouse numbers typically crash in plague years even without intervention. A better approach is to focus on reducing mouse impacts, rather than mouse numbers.
How to fool a mouse
Mouse damage to Australia’s most valuable crop, wheat, occurs mostly in the two-week period between sowing and germination. During this time, mice are attracted to the smell of the wheat germ – the nutritious and fatty part of the seed – beneath the ground, and they learn to dig up seeds with pinpoint accuracy, leading to significant crop losses.
This led to our question: can we hide the seeds so mice can’t find them?
Like many animals, mice primarily use their sense of smell to find food. The world is full of odours, and hungry foragers must prioritise important smells and disregard useless ones.
When a food is too difficult to find, or an odour is not a useful indicator of food, foragers must give up and search for something else to avoid wasting energy.
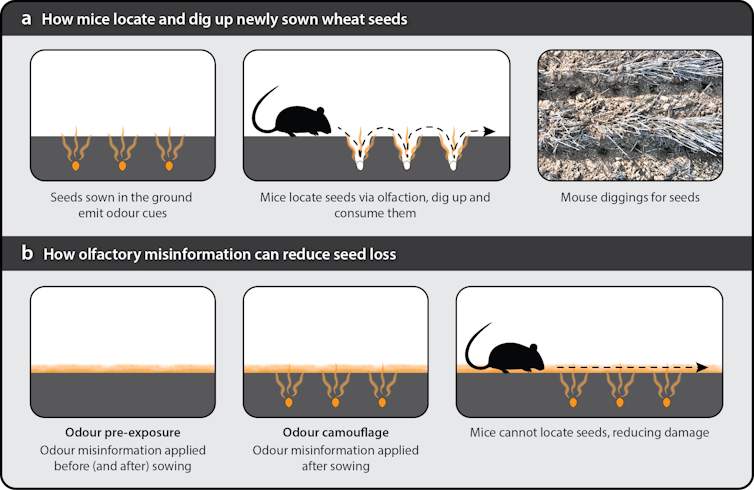
Because hungry animals can’t afford to waste effort on odours that don’t lead to food, they are vulnerable to olfactory misinformation and chemical camouflage. As with visual camouflage, if the background, in this case smell, appears the same as the item we are trying to hide, the target item cannot be distinguished.
Animals can also learn about the usefulness of information, making them vulnerable to another form of misinformation – odour pre-exposure. By deploying food odours before food is available, foragers initially attracted to the odour repeatedly receive no reward and learn to ignore it.
When the food does become available, foragers don’t follow the odours because they know they’re unrewarding. We recently used this technique to dramatically improve nest survival for threatened shorebirds at risk from by predation by invasive predators in New Zealand.
A test under tough conditions
Until now, these techniques have been tested on relatively widely dispersed food items with fewer foragers over a larger area. Whether olfactory misinformation could protect a crop with more than 300 mice and 1.6 million seeds per hectare was unclear.
We worked on a 27-hectare wheat paddock in southwest NSW, using 60 plots to test our two olfactory misinformation techniques. We used wheat germ oil to provide the odour background, as it is made from the part of wheat seeds that mice seek out and is a relatively cheap byproduct of the wheat-milling process.
Both techniques involved spraying a fine mist of wheat germ oil solution onto the plots. Each application was equivalent to the smell of around 50 times the number of seeds on the plot.
Our first technique, odour camouflage, began immediately after the crop was planted and was reapplied several times until seedlings appeared. This created a blanket of wheat odour to hide seeds from detection.
Our second technique, odour pre-exposure, had wheat germ oil applied six days before the wheat crop was planted and continued for the week after. We predicted that mice attracted to the odour before seeds were planted would begin to ignore wheat odour after repeatedly finding no seeds.
We also had three control treatments: one sprayed with canola oil to control for an oil effect, one we walked on without spraying to control for seed loss due to trampling, and one that remained totally untouched.
One and two weeks after sowing, we counted mouse damage in the form of diggings where seeds had been extracted by mice. After two weeks, we also estimated the number of seedlings that were lost to mice. The results were staggering.

After two weeks, our camouflage and pre-exposure treatments had reduced mouse damage by 63% and 74% respectively, compared to the control. We also estimated that 53% and 72% fewer seedlings, respectively, were lost to mice on these plots.
The difference in the effect of pre-exposure to wheat odour and the effect of camouflage treatments was not statistically significant, and we concluded the camouflage effect is the most likely reason for the reduction in damage.
Working with the animals
In an increasingly populated world where food security is becoming a priority, we need new ways to tackle pest problems sustainably and safely.
Our methods are simple, safe and highly effective, even during a mouse plague. They carry no risks for native wildlife and involve no killing. Mice don’t go hungry either – they simply eat the foods they ate before the wheat was planted.
We believe simple behavioural interventions like ours, which work with animals’ motivations rather than against them, are the way of the future in wildlife management and conservation.
We believe this new approach has the potential to manage pest impacts without the side effects that come from using lethal pest control.
Finn Cameron Gillies Parker receives funding from the Australian Research Council.
Catherine Price receives funding from the Australian Research Council.
Jenna Bytheway receives funding from the Australian Research Council.
Peter Banks receives funding from the Australian Research Council and The Hermon Slate Foundation. He is an external member of CSIRO's Rodent Research Advisory Panel
This article was originally published on The Conversation. Read the original article.







