Australia’s wildlife was hit hard by the 2019-20 Black Summer megafires.
Amongst the casualties were our iconic tree-dwelling koalas, with an estimated 5000 dead in New South Wales alone. They are now officially endangered in three states and territories.
In response, researchers are ramping up captive breeding to prevent extinction. Unfortunately, captive breeding faces two major challenges: it’s expensive, and it can be hard to maintain genetic diversity.
To tackle both issues, our new modelling study backs the approach of biobanking (freezing koala sperm) and tailored assisted reproduction techniques. We found these techniques would result in a five-fold decrease in the costs of running captive breeding programs.
Despite their promise, these reproductive tools have not yet become widely used in conservation. With koalas facing an uncertain future, it’s time to explore their full potential. If we get this right, we could use the same tools to help other species in rapid decline.

What are these techniques?
In animals, biobanking refers to freezing and storing sperm, eggs and embryos, as well as other cells and tissues from the body. These techniques have long been used in agriculture to store valuable sperm from top breeding bulls and crops in seed banks.
Read more: It's fish on ice, as frozen zoos make a last-ditch attempt to prevent extinction
Most people are aware of in vitro fertilisation (IVF), a common assisted reproductive technology, but other options exist such as artificial insemination and direct sperm injection into the egg. In humans, IVF and sperm injection have dramatically improved fertility while artificial insemination has revolutionised the breeding of livestock.
Models show huge drop in costs and less inbreeding
In our modelling, we set the goal of maintaining at least 90% of the genetic diversity in the captive population over a century.
We compared conventional natural breeding programs to programs mixing natural breeding with frozen koala sperm from wild animals delivered by artificial insemination or direct sperm injection.
We found supplementing captive breeding with frozen sperm would dramatically slow inbreeding rates, produce genetically healthier animals and require fewer animals to be held in breeding colonies.
To reach the genetic target, you would need 223 koalas in a conventional captive program. By contrast, adding assisted reproduction means you’d only have to keep 17 koalas.

These much smaller colony sizes are what drives down the cost. When you factor in the costs of assisted reproduction, including sperm freezing and performing artificial insemination or sperm injection, you still end up with a more than five-fold reduction in costs.
Let’s put these technologies to work
While these technologies have proven their worth for us and for livestock, we largely haven’t put them to work in wildlife recovery. We believe this is a missed opportunity to cut costs and boost genetic diversity.
Read more: To save koalas from fire, we need to start putting their genetic material on ice
The few programs which have embraced these techniques have seen success. North America’s black-footed ferret is coming back from the edge of extinction, aided in part by assisted reproduction techniques. In the 1980s, the last remaining 18 black-footed ferrets were brought into a captive breeding program in America. Because the genetic diversity was so low, researchers used artificial insemination and frozen sperm to reintroduce lost genes and reduce the damage from inbreeding.
What do we need to do?
In recent years, we’ve seen significant investment in frozen storage and genomic sequencing of tissue samples collected from wild koalas.
These technologies are useful to take stock of the genetic health of koala populations. But they can’t help us restore lost genetic diversity to wild populations because the frozen tissue samples cannot be turned into living animals.
While we’ve seen some progress in tailoring these technologies to koalas, there’s more to do. To date, 34 koala joeys have been born using artificial insemination in tame zoo koalas. These joeys, however, came from fresh or chilled sperm, not frozen. To use frozen sperm requires more research and technology development. Other procedures like embryo transfer and cryopreservation of sperm will also need more development.
Read more: Human reproductive technologies like sperm freezing and IVF could be used to save threatened species
If we perfect these techniques and technologies, we could see new possibilities for koala conservation.
These include:
- using genetic material from dead or sick koalas which would otherwise be lost
- preserving gene pools from genetically important koala populations at risk of extinction
- protecting the species against catastrophic events in the wild linked to climate change, disease and bushfire, which can cause major genetic loss
- reducing inbreeding in captive breeding programs and producing genetically fit koalas for release
- overcoming issues of separated populations and ensuring desirable breeding pairs can actually breed
- tackling relocation issues emerging from the varying diets of koalas across regions and risk of disease transfer.
We already have the expertise
Australia already has a strong network of wildlife hospitals and zoos across the koala’s range in eastern Australia, as well as existing captive colonies and technical and husbandry expertise.
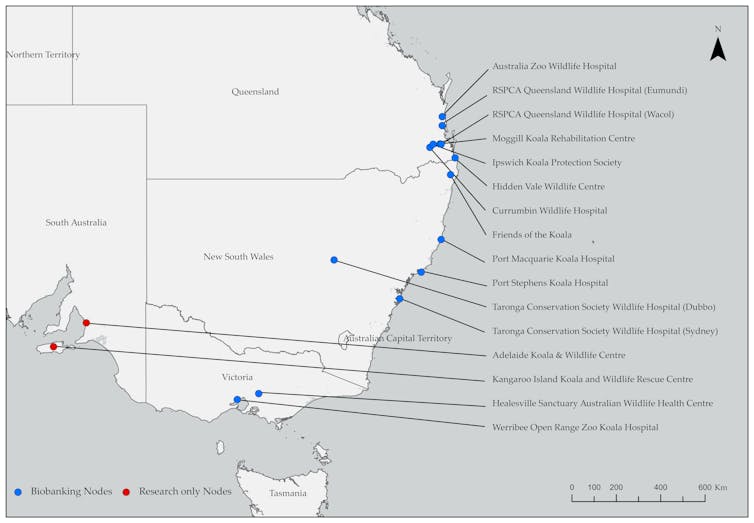
With a relatively small amount of funding (A$3-4 million to start, A$1 million annually), these sites could be equipped to collect and store koala sperm from wild populations and help perfect the technologies we need to make this a reality.
Longer term, we could adapt these technologies for other endangered marsupials. The potential is real. All we need now is attention from researchers and funding bodies.
Lachlan G. Howell is affiliated with Deakin University, the Centre for Integrative Ecology, the University of Newcastle, and FAUNA Research Alliance. Lachlan is funded by the Bushfire and Natural Hazards CRC. Lachlan is a member of the Australasian Wildlife Management Society. Lachlan would like to acknowledge several co-authors on the research study referenced in this article including Stephen D. Johnston, Justine K. O’Brien, Richard Frankham, John C. Rodger, Shelby A. Ryan, Chad T. Beranek, John Clulow and Donald S. Hudson. We also thank Port Stephens Koala Hospital and their president Ron Land for assistance in under-standing the required program costs used in the models.
Ryan R. Witt receives funding from Taronga Conservation Society Australia, the Mid North Coast Joint Organisation, WWF-Australia's Regenerate Australia Program, and the Paddy Pallin Foundation administered by the Royal Zoological Society of New South Wales. He is affiliated with the University of Newcastle and FAUNA Research Alliance. Ryan is a member of the Royal Zoological Society of New South Wales, is a scientific associate of Taronga Conservation Society Australia, and is the Australasian board member of the Companion Animals and Non-Domestic Endangered Species Committee of the International Embryo Technology Society.
John Clulow is affiliated with the University of Newcastle (Research Affiliate) and the Fauna Research Alliance.
This article was originally published on The Conversation. Read the original article.







