
With the Food and Drug Administration’s Aug. 21, 2023, approval of the first vaccine against respiratory syncytial virus, or RSV, for use during late pregnancy, the U.S. will soon have a major new tool at its disposal to protect infants against the highly contagious virus.
RSV is the most common cause of lower respiratory infections in young children and can be especially severe for infants under 6 months of age. It is the leading cause of infant hospitalization in the U.S., according to the Centers for Disease Control and Prevention. Each year, RSV is associated with half a million emergency room visits, nearly 100,000 hospitalizations and 300 deaths in young U.S. children.
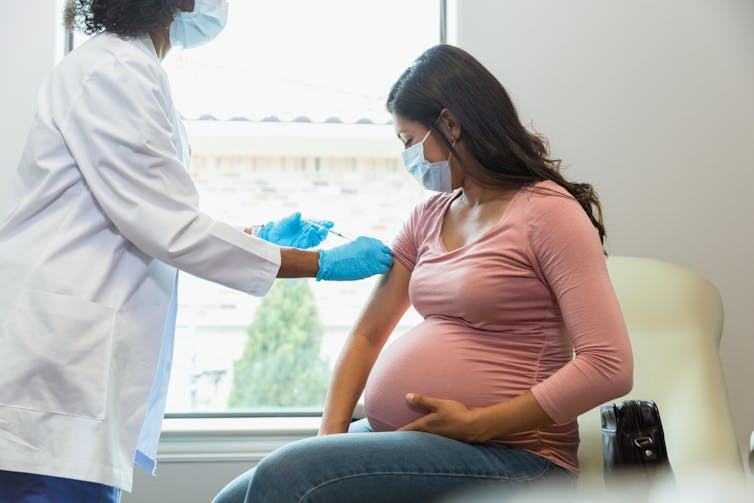
The vaccine, sold under the brand name Abrysvo, is approved for use between 32 and 36 weeks of pregnancy to protect infants from birth through 6 months of age.
The CDC plans to meet in October to set recommendations for the use of Abrysvo. That means this vaccine could become available for use during pregnancy in a matter of months.
In mid-July, the FDA also approved a long-acting, single-dose monoclonal antibody, called nirsevimab, which is sold as Beyfortus, for newborns and young children up to the age of 2 years old.
We are an infectious disease epidemiologist and pediatric infectious disease physician. We have experienced the frustration of previously limited options available for the prevention of RSV, especially during the heavier-than-usual RSV season in late 2022. The approval of a maternal vaccine and monoclonal antibody signals a major milestone in the medical profession’s ability to prevent RSV disease in children.
With these two new options soon to be available, parents of young children, along with people who are currently expecting, are likely wondering about the pros and cons of each and which to take to best protect their child from RSV.
A game-changer in the fight against RSV
The newly approved protein-based vaccine takes a similar approach as the Tdap, or whooping cough, vaccine, which is given between 27 and 36 weeks of pregnancy to protect babies against tetanus, diphtheria and pertussis (whooping cough). Abrysvo stimulates the mother’s immune system to produce antibodies that cross the placenta and offer protection to the newborn against RSV illness, starting at birth.
The FDA based its approval on clinical trial data from more than 7,000 participants across 18 countries who either received the RSV vaccine between 24 and 36 weeks of pregnancy or received a placebo shot. In the trial, the maternal RSV vaccine prevented 82% of severe lower-respiratory illnesses caused by RSV in infants in the first 3 months of life, and 69.4% through 6 months of age.
While there were no vaccine-related safety concerns raised in the trial, including preterm birth, low birth weight, birth defects, developmental delay or death, the vaccine will come with a warning about a less-than-1% increase in preterm birth that was seen in the group that received the RSV vaccination in the clinical trial. There is currently no proof that the vaccine is causally linked with preterm birth, and the 1% increase was not significant.
The FDA also requires the vaccine manufacturer to continue monitoring the safety of the vaccine for use during pregnancy.
Abrysvo was also approved by the FDA in May 2023 to prevent RSV illness in adults 60 years and older.
Monoclonal antibodies also provide protection
For those who are unable to get the RSV vaccine during their pregnancy, there is also an option to provide ready-made antibodies to protect the baby.
Nirsevimab, also known as Beyfortus, is a monoclonal antibody approved for babies up to 8 months of age during the RSV season and children up to 24 months of age who are at high risk of severe RSV. Beyfortus is given as a single shot of laboratory-made human antibodies. These antibodies help protect against lower-respiratory tract disease, including bronchiolitis and pneumonia, caused by RSV.
Clinical trial data from 350 sites across 31 countries showed that Beyfortus was 75% effective against RSV-associated lower respiratory illness and 62% effective against RSV-associated hospitalization in the first 5 months after birth. Mild adverse reactions associated with Beyfortus included rashes and swelling or pain at the place where the injection was made.
There are some children who should not receive Beyfortus or should be cautious about receiving Beyfortus, including those with a history of serious reactions to the ingredients in that medication and children with bleeding disorders.
Parsing the differences
Both the maternal vaccine and the monoclonal antibody have been shown to work in reducing the risk of severe RSV disease in young infants, and the efficacy and duration of protection appears to be similar. Clinical trials showed that the vaccine was protective up to 6 months of age and the antibody up to 5 months of age.
While Abrysvo stimulates the production of the mother’s own antibodies that get passed on to the baby, Beyfortus is not actually a vaccine. It instead provides ready-made antibodies given as an injection to protect the child. Beyfortus will go to work immediately after administration, and babies of mothers who are vaccinated during pregnancy will be protected from birth, but Abrysvo takes approximately 14 days after the shot to build up effective antibodies in the mother. The vaccine should be taken at least 14 days before expected delivery – and ideally even before then – in order to adequately protect the baby.
Both the vaccine and the monoclonal antibody target the F-protein of the virus, the protein that helps the virus enter cells and spread infection. However, the vaccine creates antibodies that target all sites on the F-protein, while Beyfortus antibodies target a single site – known as “site zero” – of the F-protein. Both result in passive immunity to the baby, providing protection during a time that babies are most susceptible to severe RSV disease.
When mothers are vaccinated within the specified window and babies are born at term, the protection from Abrysvo is sufficient for the babies. When the mother is not vaccinated in pregnancy, then Beyfortus is available for infants from birth.
Another big difference between the two products is cost. Pre-prepared antibodies like Beyfortus can be expensive to produce and carry a higher cost compared to the Abrysvo vaccine – about US$395 to $500 per Beyfortus shot compared to $180 to $295 per Abrysvo shot. The cost of Abrysvo and how it will be covered by insurance will depend on what the CDC says in October. Regardless, both shots need to be given by a health care professional, which will require a medical visit.
While both provide a substantial opportunity to prevent severe illness associated with RSV in newborns and young infants, most children will not need both.
In special cases, Beyfortus could be offered to an infant of a mother who received the vaccine. For example, this might be appropriate if birth occurs less than 14 days after the administration of the vaccine, or if the baby is born prematurely. In addition, the monoclonal antibody can be given to protect infants with high-risk conditions for RSV, such as immune deficiency and chronic lung or heart disease, through their second year of life.
The bottom line
Both products are safe and effective, and it is important to protect young infants and children at risk from RSV.
Until now, effective monoclonal antibodies were only available for the most premature babies. But many of the infants who get RSV are born full term.
Families should discuss their options for RSV prevention with their pregnancy care provider and pediatrician.
Annette Regan receives funding from the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the US Centers for Disease Control and Prevention, and the Pan American Health Organization.
Flor M. Munoz is a member of the data safety committee or advisory boards to Pfizer, Sanofi, AztraZeneca, GSK, Moderna, and Meissa vaccines. She receives research funding from the National Institutes of Health, the US Centers for Disease Control and Prevention, Pfizer and Gilead. She is a member of the board of the National Foundation of Infectious Diseases (NFID).
This article was originally published on The Conversation. Read the original article.







