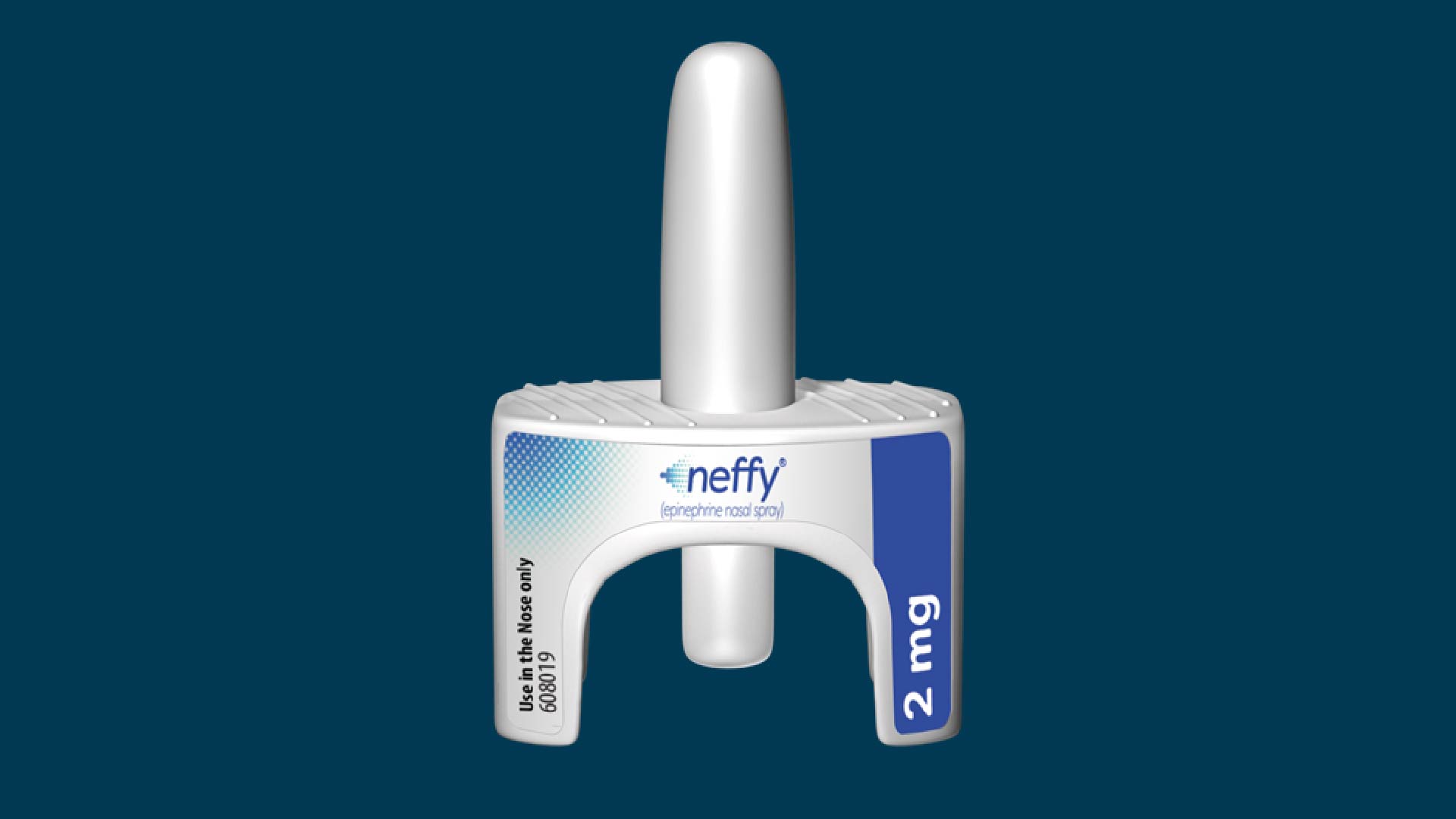
The Food and Drug Administration (FDA) has approved the first needle-free treatment for the life-threatening allergic reaction anaphylaxis.
In a statement released Friday (Aug. 9), the FDA announced it had approved Neffy, a nasal-spray formulation of epinephrine. That's the same medication found in EpiPens.
Also known as adrenaline, epinephrine is a chemical made by the body that plays a role in its fight-or-flight response. Epinephrine treats allergic reactions because it rapidly relaxes the muscles of the airways, preventing spasms and opening up passageways needed for breathing. In addition, it helps reverse a dangerous drop in blood pressure that can be triggered by severe allergies in part by constricting blood vessels.
These dangerous effects unfold during anaphylaxis, a sudden and life-threatening allergic reaction that affect the whole body. And now, there will be a needle-free option to treat the condition.
Related: Could allergies be 'deleted' someday?
"Today's approval provides the first epinephrine product for the treatment of anaphylaxis that is not administered by injection," Dr. Kelly Stone, the associate director of the Division of Pulmonology, Allergy and Critical Care in the FDA's Center for Drug Evaluation and Research, said in the statement.
"The availability of epinephrine nasal spray may reduce barriers to rapid treatment of anaphylaxis," Stone said. "As a result, neffy provides an important treatment option and addresses an unmet need."
Neffy is approved for adult and pediatric patients who weigh at least 66 pounds (30 kilograms). It's a single-dose spray intended to be administered into one nostril. Similar to products like EpiPens, a second dose of the spray can be given if a person's symptoms do not improve. The second dose would require a second Neffy spray bottle and it would be given in the opposite nostril as the first dose.
"Patients may need to seek emergency medical assistance for close monitoring of the anaphylactic episode and in the event further treatment is required," the FDA notes.
Some medical conditions, including a history of nasal surgery, may affect how well Neffy absorbs through the tissues of the nose. Because of that, the FDA says individuals with such medical histories should talk to a health care provider before using Neffy.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!







