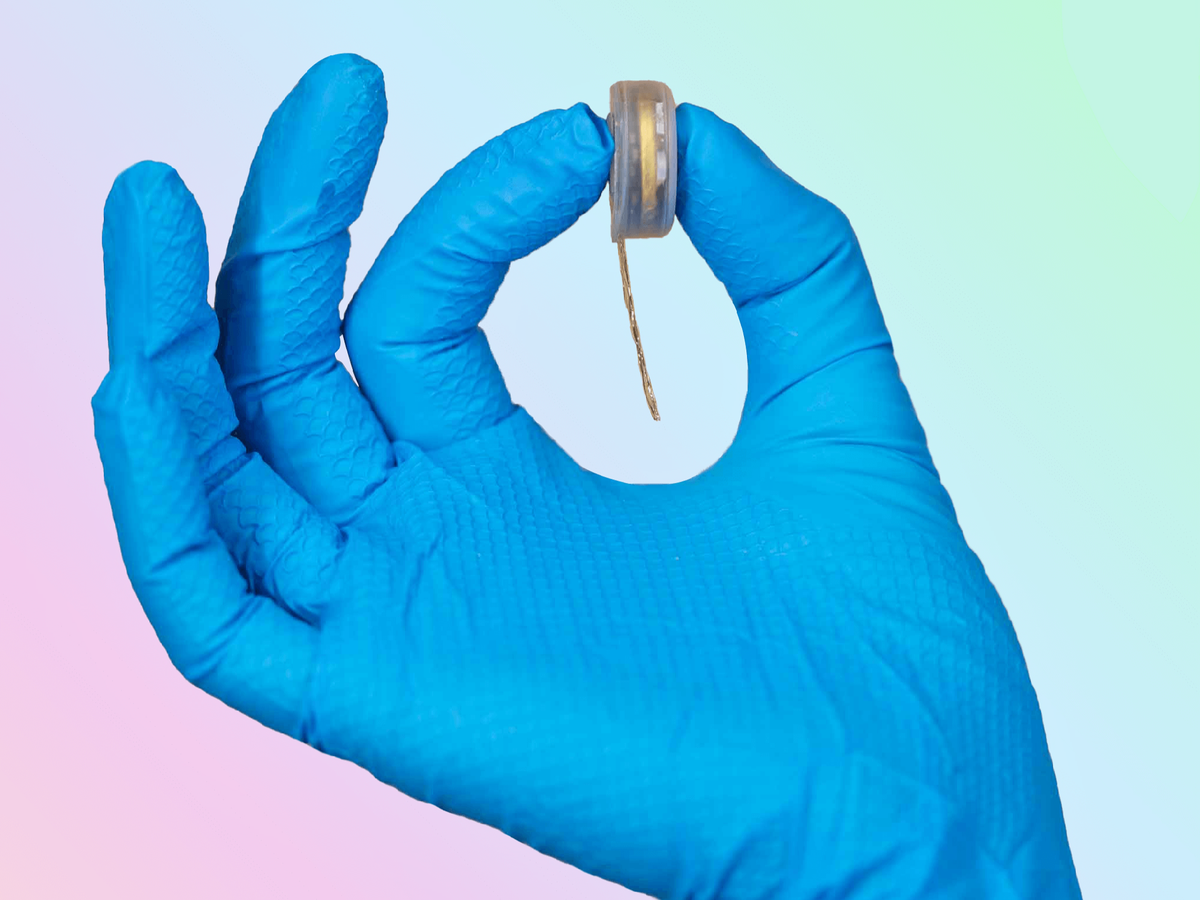
Elon Musk’s brain chip company Neuralink said on Thursday that it now has regulatory approval in the US to test its implants in human subjects.
Neuralink has been building chips to be implanted into the skull for a brain-computer interface, claiming their development has the potential to help restore vision in the blind, and even help paralysed people walk again.
While Mr Musk has said on several occasions since 2019 that the company is ready to go for experiments in humans to treat paralysis and blindness, the US Food and Drug Administration (FDA) had however rejected proposals by the company to begin human clinical trials.
For instance, in early 2022, when Neuralink applied for human testing, the FDA rejected the proposal saying there were “dozens of issues the company must address”.
These issues flagged by the FDA included the use of lithium batteries in Neuralink’s device and the likelihood of the implant’s wires interfering with the brain.
The federal agency also expressed concerns about whether the firm’s implants can be removed without causing brain damage.
Neuralink has also come under the scanner of at least one US government probe after animal rights watchdogs accused the firm of “inadequate care” of its research monkeys.
Reuters previously reported that the brain chip company killed about 1,500 animals, including over 280 sheep, pigs and monkeys since 2018.
The US Department of Agriculture also began investigating Neuralink’s potential violations of the Animal Welfare Act.
Responding to the animal abuse complaints, the company said in a blogpost that it was “absolutely committed to working with animals in the most humane and ethical way possible”.
“The use of every animal was extensively planned and considered to balance scientific discovery with the ethical use of animals,” it said.
In a tweet in November last year, Mr Musk said he was confident that Neuralink’s device was “ready for humans”, adding that the timing for beginning human trials was a “function of working through the FDA approval process”.
“We want to be extremely careful and certain that it will work well before putting a device into a human but we’ve submitted I think most of our paperwork to the FDA and probably in about six months we should be able to upload Neuralink in a human,” the Tesla and SpaceX chief said.
On Thursday, the company said it has finally received approval from the federal agency to begin trials in humans.
“We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!” the brain chip company said.
The FDA approval “represents an important first step that will one day allow our technology to help many people”, Neuralink said, adding that it would soon announce more information on the recruitment of people for clinical trials.
FDA hasn’t immediately responded to The Independent‘s queries on the Neuralink approval claim.



.jpg?w=600)


