
In recent years, scientists have shown that a type of man-made chemicals called per- and polyfluoroalkyl substances, or PFAS, can linger in our bodies and may lead to health risks such as certain cancers, developmental delays in children, and an impaired immune system.
But they’re everywhere: Manufacturers have long put PFAS in a wide range of items, such as cookware, clothing, and cleaning products, because they’re durable, water- and oil-resistant, and can put out fires.
When we use these products, PFAS leach into the environment through our wastewater and can stick around for hundreds of years. In fact, most people in the United States have the chemicals in their blood, according to the Centers for Disease Control and Prevention.

To ensure that drinking water is safe for consumption, the U.S. Environmental Protection Agency is proposing the first-ever federal restrictions on six “forever chemicals” known to harm human health. The agency will hold a public comment session on May 4 and expects to finalize the regulation by the end of this year.
Previously, the EPA recommended limiting the levels of two varieties of PFAS — perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) — to 70 parts per trillion in drinking water. Now, the agency wants to mandate stricter levels: 4 ppt for PFOA and 4 ppt for PFOS. Four other kinds of PFAS will also be regulated on a proposed “hazard index” to determine their cumulative risk.
“EPA anticipates that if fully implemented, the rule will prevent thousands of deaths and reduce tens of thousands of serious PFAS-attributable illnesses,” the agency wrote in a statement.
Scientists and environmental groups are praising the proposal, which they say is long overdue. But setting limits is just half the battle: Scientists are now hunting for ways to filter and destroy the chemicals before they can make it into our water.
"We really need to double down and see what we can do from a chemistry and materials standpoint: what we can invent to more effectively remove and destroy these harmful molecules from the environment," Leslie Hamilton, a materials scientist at the Johns Hopkins University Applied Physics Laboratory, tells Inverse.
Imperfect capture

PFAS are used in a wide range of industrial and consumer products, including firefighting foam, non-stick cookware, and waterproof clothing.
These chemicals range in length between four and 12 molecules. Scientists refer to PFAS compounds with six or more carbons as long-chained, while short-chained typically have around seven carbons. The latter were rolled out by plastics manufacturers in the early 2000s as a safer alternative to older generations of long-chained chemicals.
But these newer PFAS have demonstrated significant health risks. (PFHxS and PFBS, both among the six mentioned by the EPA, are short-chained.)
While some federal and state regulations have led manufacturers to phase out certain kinds of PFAS, concern has grown over the potential health effects of exposure once those chemicals make their way into nature.
“I think a lot of these new regulations stem from the fact that we are learning that there is very little ‘safe’ quantity of PFAS,” Danielle Nachman, a project manager in charge of PFAS research at APL, tells Inverse.
The EPA’s new proposal demands that companies stop discharging PFAS into waterways, but municipal water companies will likely bear the brunt of the chemical crackdown. If finalized, the proposal would require that water utilities monitor for six PFAS chemicals, notify the public when limits are exceeded, and remove them.
Along with the newly proposed limits, the White House Office of Science and Technology Policy (OSTP) released a report describing the state of science on PFAS. The OSTP laid out the current standard techniques for capturing PFAS from water: granulated active carbon filters, ion exchange resins, and reverse osmosis membranes.
Carbon filters are the least expensive, but they don’t catch everything and can clog up with other contaminants, so they require lots of maintenance. Resins and membranes may capture PFAS more selectively, but they get expensive — especially for large-scale use.
Even if a treatment plant gets most PFAS from drinking water, it must be destroyed responsibly. Today, incineration is the best option, but shorter PFAS can form from incomplete combustion. And burning PFAS releases fumes and airborne health risks, which travel miles away.
“These current processes are good, but they need to be better,” Nachman says.
Breaking the bonds
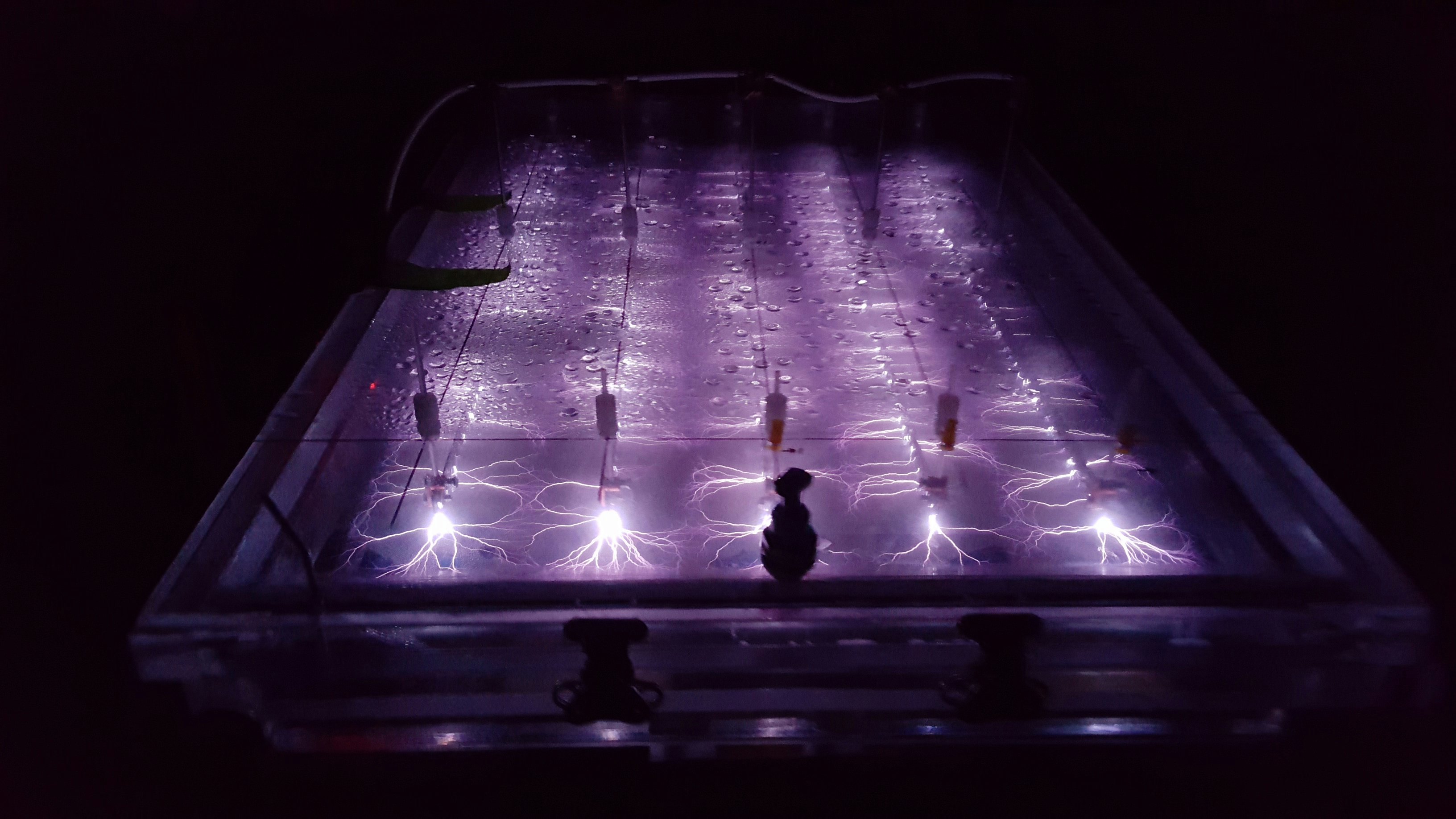
In search of a better way to degrade PFAS, the Air Force has tested a high-tech plasma reactor. Other labs are looking into less energy-intensive techniques, like biological degradation with enzymes or chemical degradation at low temperatures. But according to the OSTP report, this tech hasn’t been demonstrated at large enough scales, may work too slowly, and could require tricky pH levels.
“Destroying carbon-fluorine bonds is extremely, extremely challenging,” Nachman says. “It's one of the biggest challenges that we have in chemistry.”
Nachman’s group at APL is working on some innovative projects, including “nanowhiskers” attached to aluminum-based membranes. The “whisker” molecule is like a chain: One end likes water, the other repels it, and together, they attract contaminants like PFAS.
APL has found that nanowhiskers outperform active carbon filters by more than ten-fold in catching PFOA and PFOS. What’s more, the coating is designed to be cost-effective.
Another PFAS-destroying technique combines chemicals and UV light to break down PFAS into less harmful compounds. To this end, the APL team wants to use iron-based nanoparticles and UV light to create reactive oxygen species and destroy PFAS in just minutes of operation at practical pH levels. “They'll go and chew up whatever is around. They’re just really reactive,” Nachman says.
The team thinks it’s a promising early step to shattering carbon-fluorine bonds and cleaning drinking water.
While there are over 9,000 different PFAS molecules, which vary in size and threat, leading experts think they should all be managed uniformly as a class that includes both long- and short-chained chemicals . “Regardless of EPA regulations, we're striving to destroy or capture 100 percent,” Nachman says.
In addition to scaling up tech and lowering costs, Nachman says it’s also crucial to design PFAS-degrading processes sustainably from the start. “We want to utilize materials that are environmentally friendly, that are cheap and easy to use,” Nachman says. “We don't want to create more contamination from the contamination.”







