Scientists have been working to understand the root causes of dementia and Alzheimer’s disease for decades now. But one of the reasons we don’t yet have a cure for this disease is because of the complexity of the human brain – alongside the complexity of the disease itself.
One of the leading theories in the field suggests that Alzheimer’s disease is caused by the abnormal accumulation of two proteins called amyloid beta and tau in the brain, resulting in plaques and tangles. Amyloid plaques are clumps that form between neurons, which can damage surrounding cells, while tau tangles block communication between nerve cells.
Read more: Alzheimer's disease: surprising new theory about what might cause it
For years now, scientists have been trying to understand how the accumulation of these proteins begins and how this affects brain health, leading to memory loss. Despite the huge amount of research that’s happened to date, there’s not been much success in treating and preventing Alzheimer’s disease.
This has led many experts in the field to wonder whether there’s something else we should also be looking at in the brain when it comes to understanding and curing Alzheimer’s disease.
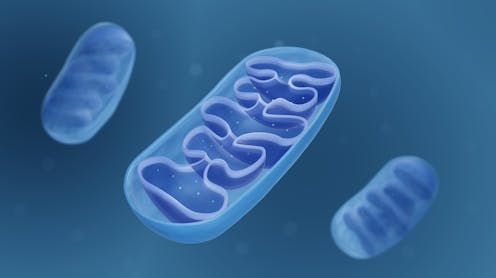
A recent article in New Scientist describes an idea which could be important in the field of brain health. This article highlights an alternative theory: that damage to mitochondria (the energy-producing structures within cells) could actually be the cause of Alzheimer’s.
Energy deficit
Mitochondria are found in virtually all the body’s cells. They use both oxygen and breakdown products from food to make a high energy molecule known as adenosine triphosphate (ATP). ATP is like your cells’ energy currency – kind of like a rechargeable battery. Our cells use ATP for the energy needed to carry out everyday functions and maintain their own health. Once used up, mitochondria can reload it with energy.
Mitochondria also have a host of other functions important for cellular health, such as telling the cell’s nucleus (the cell’s hub of genetic information) to carry out important functions, and sending signals to other cells. They’re also packed full of antioxidants – molecules that protect cells from damage.
Mitochondria are particularly important for the brain. The human brain only accounts for around 2% of our total body weight, yet even at rest, the brain uses around 20% of the body’s total energy expenditure. As the control centre of the body, the brain needs this energy in order to carry out its many important functions which make virtually everything we do possible – whether that’s blinking, smiling or memorising a poem.
So, our brain cells – particularly our neurons, the brain cells that send and receive signals from our brain to the rest of the body – have high energy needs. This is why each neuron can contain thousands of mitochondria.
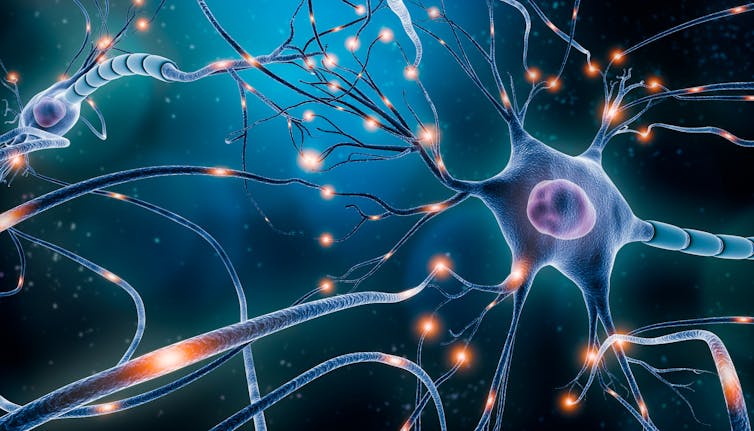
It’s thought that neurons are formed at birth and do not get regenerated at any point in a person’s life. Instead, their mitochondria and cellular parts are constantly turning over and being renewed. This ensures that their mitochondria remain healthy – which in turns ensures the neuron can function properly. Essentially, this means that as long as the mitochondria are healthy, the neuron is too.
But what would happen if the mitochondria stopped being able to produce enough energy for our cells to carry out their functions and repair damage? This would mean the cells may start to accumulate damage. In neurons, this could result in damage – and even death.
This is the foundation of the mitochondrial cascade hypothesis.
Mitochondrial loss
The mitochondrial cascade hypothesis was actually first published by scientist and clinician professor Russell Swerdlow in 2004. This landmark article reviewed numerous studies which had previously found evidence of mitochondrial damage in Alzheimer’s disease. In the paper, Swerdlow proposed a new theory suggesting that problems with mitochondria and their function could provide an alternative explanation for why Alzheimer’s disease develops.
However, despite increasing evidence showing mitochondrial loss in the neurons of patients with Alzheimer’s, the idea that mitochondrial dysfunction could be a cause has remained on the fringes of dementia research. There are many reasons why this is the case.
First, a large proportion of the limited funding given to dementia research in the past few decades has gone to scientists studying amyloid beta and tau. This was thanks to promising studies in the field which suggested that removing or reducing the amount of amyloid beta and tau in the brain could have an effect on cognitive function.
Second, until relatively recently the methods used to study mitochondria in humans have been limited – meaning that we’ve also been limited in our ability to detect, prevent or cure mitochondrial dysfunction. But developments in the field may soon make it possible to transfer healthy mitochondria into cells. This could therefore allow us to study what would happen if we replaced damaged mitochondria in the neurons of patients with Alzheimer’s disease.
But while it’s clear that problems with the brain’s mitochondria are linked to neurodegenerative diseases, there are still many questions we need to answer before we can start developing treatments. For example, we need to understand what damages the brain’s mitochondria, and how to prevent this damage.
Dementia is a complex disease. This may mean there isn’t a one-size-fits-all cure for it. It could be the case that we may need to target multiple different mechanisms in order to treat the disease.
Afshan Malik receives funding from the charity Alzheimer's Research UK
This article was originally published on The Conversation. Read the original article.







